Mechanism of strand exchange of eukaryotic homologous recombination from RAD51 D-loop structures
Luo, S.C.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA repair protein RAD51 homolog 1 | 361 | Mus musculus | Mutation(s): 2 Gene Names: Rad51, Rad51a, Reca EC: 3.6.4 | 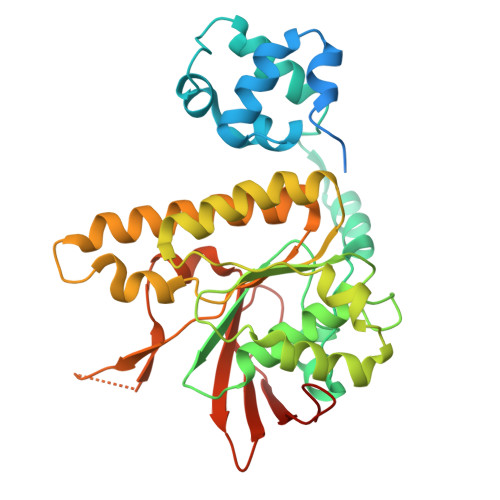 | |
UniProt | |||||
Find proteins for Q08297 (Mus musculus) Explore Q08297 Go to UniProtKB: Q08297 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q08297 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (27-MER) | A [auth L] | 27 | Mus musculus | 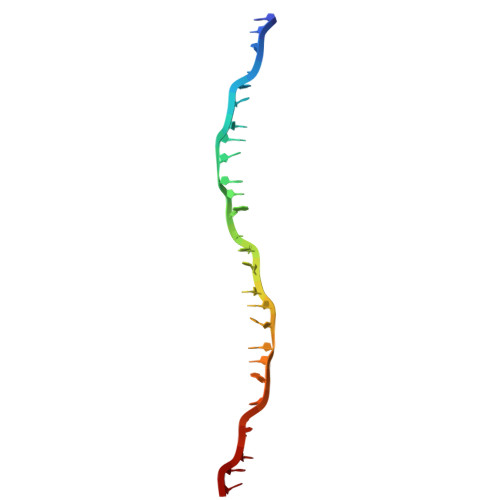 | |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ANP Query on ANP | AA [auth G] K [auth C] M [auth B] O [auth A] R [auth I] | PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER C10 H17 N6 O12 P3 PVKSNHVPLWYQGJ-KQYNXXCUSA-N |  | ||
| MG Query on MG | BA [auth G] L [auth C] N [auth B] P [auth A] Q [auth F] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.21.2_5419: |
| RECONSTRUCTION | cryoSPARC |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Academia Sinica (Taiwan) | Taiwan | AS-TP-113-L01 to M.C.H |