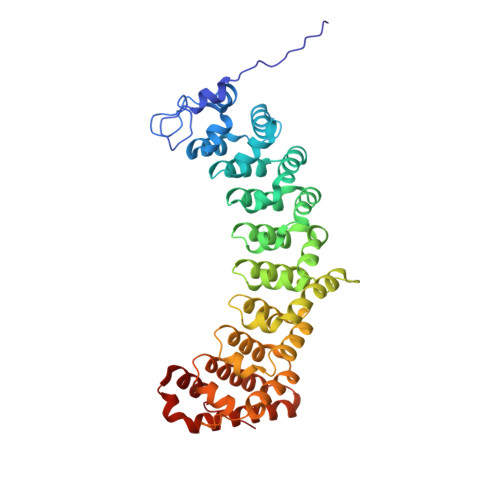
Crystal structures of Caenorhabditis elegans PUF-3 depict plasticity of RNA recognition that enables germline gene regulation.
Zhang, Y., Kennedy, F.D., Hall, T.M.T.(2026) Nucleic Acids Res 54
- PubMed: 41495898
- DOI: https://doi.org/10.1093/nar/gkaf1431
- Primary Citation of Related Structures:
9PMM, 9PQV, 9PQW, 9PSA, 9PSB, 9PSC, 9PTD, 9PTG, 9PTJ - PubMed Abstract:
Prototypical PUF proteins are known for modular, sequence-specific RNA recognition. A single PUF protein in Drosophila melanogaster Pumilio and two closely related proteins in mammals, PUM1 and PUM2, are representative. In contrast, Caenorhabditis elegans PUF proteins have evolved to include four subfamilies of single-stranded RNA-binding proteins that recognize distinct sequence elements. Here, we provide an in-depth structural and biochemical analysis of C. elegans PUF-3, a member of the subfamily lacking structural information. We determined crystal structures of PUF-3 in complex with RNAs representing three classes of binding elements. These structures and quantitative RNA-binding assays fine-tune the features of PUF-3 RNA recognition and demonstrate how PUF-3 binds to varied target RNA elements by accommodating divergent sequences between conserved features. Our analyses here and published biological data suggest that PUF-3 may redundantly regulate RNA targets with other PUF proteins. We determined the in vitro binding affinities of C. elegans PUF protein subfamily representatives for the range of corresponding RNA elements. We find that PUF-3 and FBF-2 have broader RNA recognition, whereas PUF-8 and PUF-6 are highly selective. These subfamily recognition properties and protein expression patterns support a working model for PUF protein activities in different regions of the C. elegans germline.
- Epigenetics and RNA Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, United States.
Organizational Affiliation: