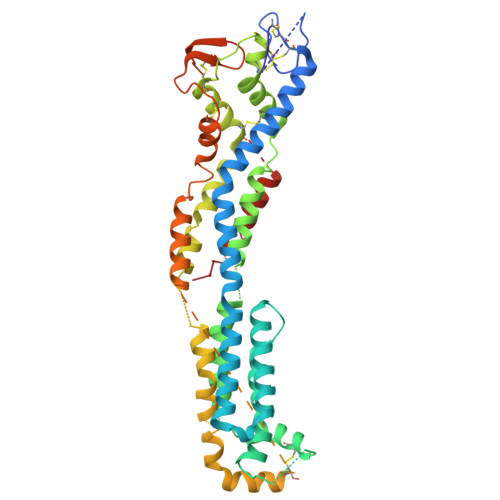
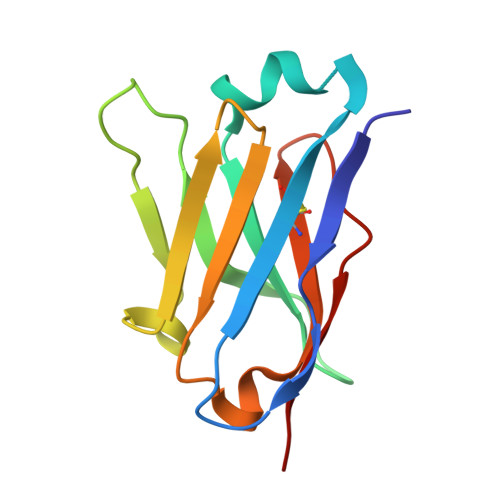
Identification and non-clinical characterization of SAR444200, a novel anti-GPC3 NANOBODY® T-cell engager, for the treatment of GPC3+ solid tumors.
Meoni, P., Vintem, A.P.B., Cortez-Retamozo, V.F., Jacobs, J., De Tavernier, E., Fiorentini, P., Van Hoorick, D., Batchelor, J.D., Svidritskiy, E., Qiu, Y., Dejonckheere, E., Li, A., Pao, L.I., Buyse, M.A.(2025) Mol Cancer Ther
- PubMed: 41041827
- DOI: https://doi.org/10.1158/1535-7163.MCT-24-1049
- Primary Citation of Related Structures:
9BDO, 9NRJ, 9NTQ, 9NTT - PubMed Abstract:
T-cell engager (TCE) immunotherapy has demonstrated significant clinical activity in multiple cancers by inducing co-engagement of T-cells and tumor cells, resulting in T-cell activation and T-cell-dependent cellular cytotoxicity (TDCC) against tumor cells. Current-generation TCEs are predominantly composed of antibody-based binding domains targeting the CD3e molecule of the T-cell antigen receptor (TCR)/CD3 complex on T-cells and a tumor-associated antigen on tumor cells. However, limitations of this approach include cytokine release syndrome and a limited therapeutic window. Here, we report the generation and preclinical evaluation of SAR444200, the first NANOBODY®-based TCE clinical candidate binding to TCRαβ and GPC3 to co-engage T-cells and GPC3+ tumor cells, causing TDCC. SAR444200 bound with nanomolar to picomolar affinity to TCRαβ and GPC3 respectively and induced in vitro TDCC against multiple human tumor cell lines with differential GPC3 expression with picomolar potency. In vivo analysis using human cancer cell line-derived (HuH-7 and HepG2) xenografts in immunodeficient mice showed complete tumor regression at doses starting from 0.7 mg/kg. In exploratory non-human primate studies, intravenous administration of SAR444200 was well tolerated up to 8 mg/kg and exhibited greater than dose-proportional clearances and dose-proportional maximum concentrations across the tested dose range. The highly potent and efficacious activity of SAR444200 in diverse models of GPC3+ tumors and the extremely wide tolerated dose range merits further development of this compound. Furthermore, NANOBODY®-based TCEs developed using an anti-TCRαβ moiety may have specific advantages for the development of TCEs.
- Sanofi (Belgium), Ghent, Belgium.
Organizational Affiliation: