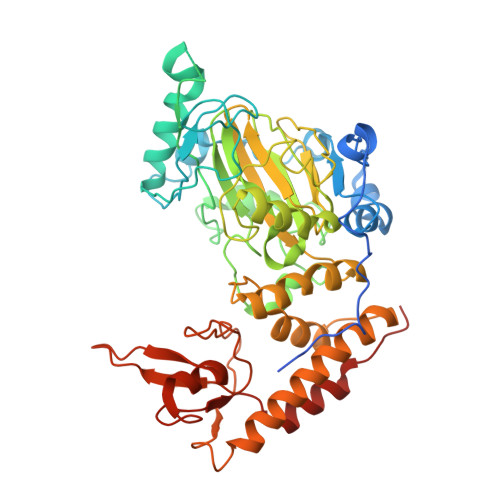
Structural mechanism of H3K27 demethylation and crosstalk with heterochromatin markers.
Lin, C.C., Zhao, Y., Foley, C.A., Hawkins, A.T., James, L.I., Frye, S.V., McGinty, R.K.(2025) Mol Cell 85: 2869-2884.e6
- PubMed: 40683254
- DOI: https://doi.org/10.1016/j.molcel.2025.06.025
- Primary Citation of Related Structures:
9NQU - PubMed Abstract:
Histone H3 lysine 27 trimethylation (H3K27me3) is a repressive histone modification that is a hallmark of facultative heterochromatin. H3K27me3 is installed by the polycomb repressive complex 2 (PRC2) and removed by KDM6 family Jumonji C (JmjC) domain demethylases. Structural studies have elucidated how PRC2 functions on nucleosomes and its regulation by local histone modification signatures. However, the molecular mechanisms governing H3K27 demethylation to reactivate silenced chromatin remain poorly understood. Here, we report the cryoelectron microscopy (cryo-EM) structure of mouse KDM6B bound to the nucleosome. Our structure shows how KDM6B engages wrapped nucleosomal DNA together with both extranucleosomal DNA linkers to position its catalytic JmjC domain for H3K27 demethylation. KDM6B induces an overlapped linker DNA conformation consistent with function in a compact chromatin environment. We further show that linker histones and H2AK119ub1, both enriched in heterochromatin, antagonize KDM6B function, suggesting that linker histone eviction and H2A deubiquitylation precede H3K27 demethylation during heterochromatin activation.
- Center for Integrative Chemical Biology and Drug Discovery, Division of Chemical Biology and Medicinal Chemistry, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Organizational Affiliation: