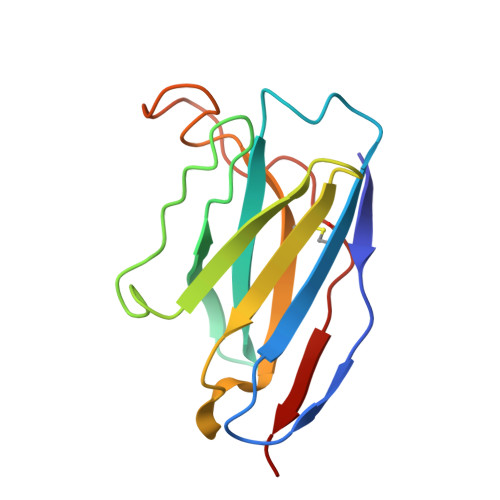
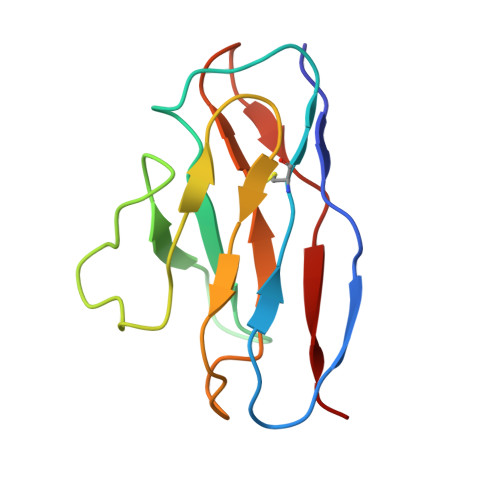
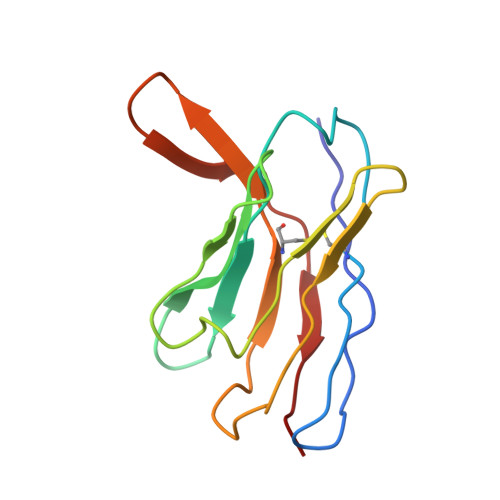
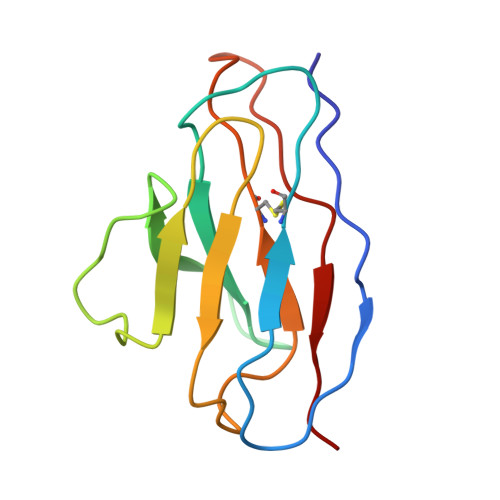
A distinctive IGHV3-66 SARS-CoV-2 neutralizing antibody elicited by primary infection with an Omicron variant.
Fan, Q., Liu, C., Guo, H., Tang, S., Wang, H., Zhou, B., Sun, Y., Wang, M., Ge, X., Liu, L., Ju, B., Zhang, Z.(2025) Structure 33: 1165-1177.e6
- PubMed: 40306272
- DOI: https://doi.org/10.1016/j.str.2025.04.005
- Primary Citation of Related Structures:
9LBS - PubMed Abstract:
SARS-CoV-2 Omicron sub-variants continuously evolve under the pressure of neutralizing antibodies (nAbs), eliminating numerous potential elite monoclonal nAbs. The IGHV3-53/3-66 public nAbs have great potential for neutralizing SARS-CoV-2. However, it has been unclear whether a primary Omicron infection could also induce IGHV3-53/3-66 nAbs. In this study, we report an IGHV3-66-encoding monoclonal nAb, ConBA-998, that was elicited by primary infection with BA.1. ConBA-998 is an Omicron-dependent nAb with high binding affinity that triggers the shedding of the S1 subunit from the spike protein. The cryo-electron microscopy (cryo-EM) structure revealed the interactions between ConBA-998 and the Omicron BA.1 spike protein. ConBA-998 has a distinctive binding mode to receptor-binding domain (RBD) that differs from canonical IGHV3-53/3-66 nAbs. Overall, our findings indicate that Omicron may elicit unique specific nAbs distinct from those induced by pre-Omicron variants, providing further insights into SARS-CoV-2 variant-specific antibody responses.
- The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Institute for Hepatology, National Clinical Research Center for Infectious Disease, Shenzhen Third People's Hospital, Shenzhen, Guangdong Province 518112, China.
Organizational Affiliation: