Structural study of Semliki Forest virus through Cryo-EM
Jia, X., Li, S., Yu, H., Zhang, Q., He, J.(2025) Dian Zi Xian Wei Xue Bao 44: 1
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Envelope glycoprotein E2 | A [auth k], D [auth b], G [auth e], J [auth h] | 422 | Semliki Forest virus | Mutation(s): 0 Membrane Entity: Yes | 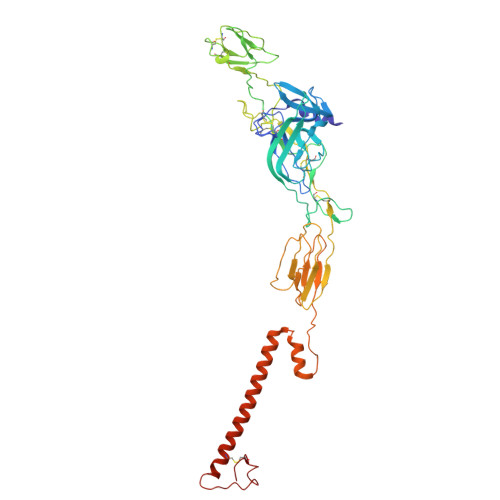 |
UniProt | |||||
Find proteins for P0DJZ6 (Semliki forest virus) Explore P0DJZ6 Go to UniProtKB: P0DJZ6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0DJZ6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Capsid protein | B [auth l], E [auth c], H [auth f], K [auth i] | 267 | Semliki Forest virus | Mutation(s): 0 EC: 3.4.21.90 Membrane Entity: Yes | 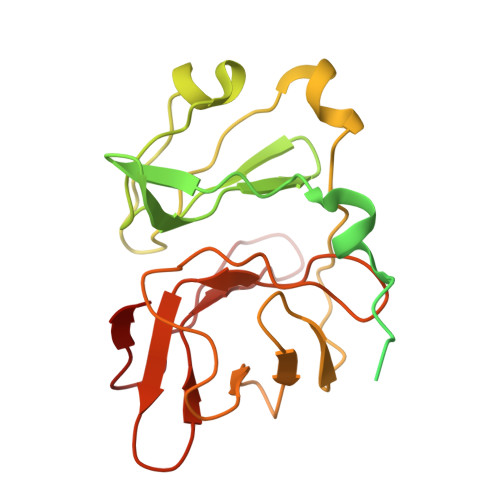 |
UniProt | |||||
Find proteins for P0DJZ6 (Semliki forest virus) Explore P0DJZ6 Go to UniProtKB: P0DJZ6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0DJZ6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Spike glycoprotein E1 | C [auth j], F [auth a], I [auth d], L [auth g] | 438 | Semliki Forest virus | Mutation(s): 0 Membrane Entity: Yes | 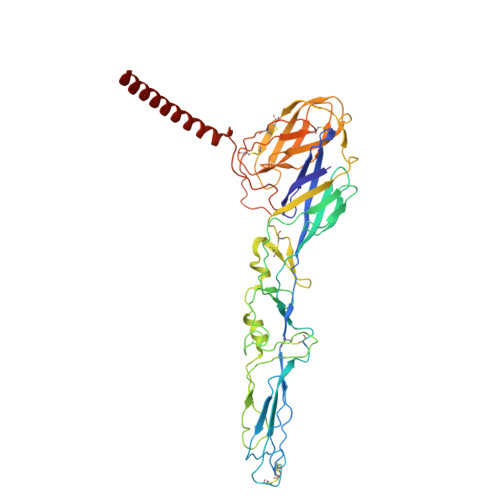 |
UniProt | |||||
Find proteins for P03315 (Semliki forest virus) Explore P03315 Go to UniProtKB: P03315 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P03315 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other government | China | 2021B1212040017 |