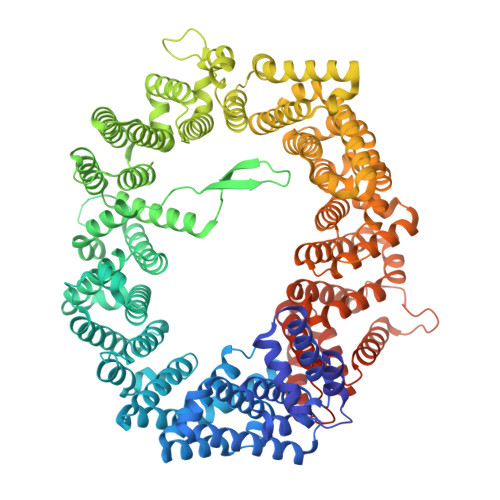
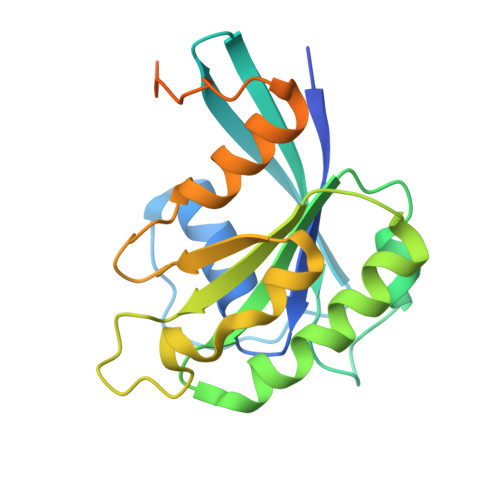
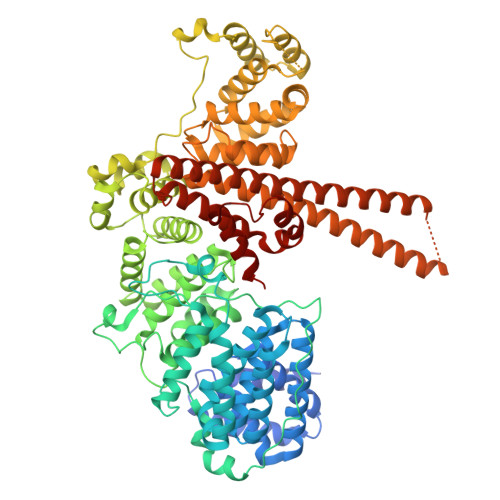
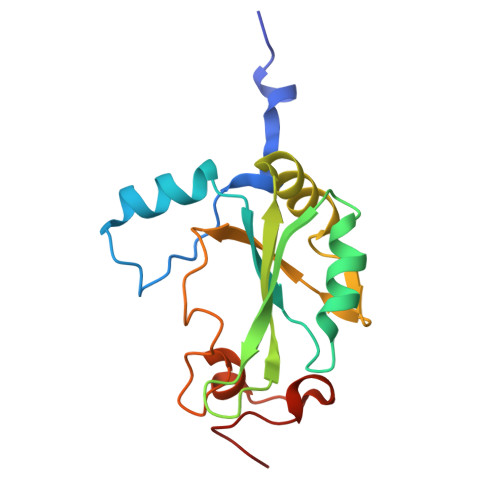
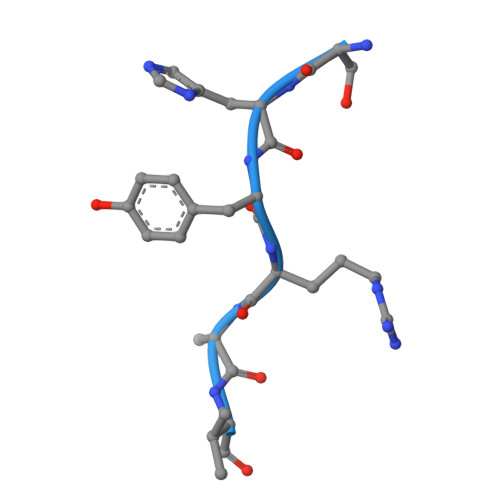
Structural basis for the synergistic assembly of the snRNA export complex.
Dubiez, E., Garland, W., Finderup Brask, M., Boeri Erba, E., Heick Jensen, T., Kadlec, J., Cusack, S.(2025) Nat Struct Mol Biol 32: 1555-1566
- PubMed: 40610714
- DOI: https://doi.org/10.1038/s41594-025-01595-5
- Primary Citation of Related Structures:
9HFL - PubMed Abstract:
The nuclear cap-binding complex (CBC) and its partner Arsenite-Resistance Protein 2 (ARS2) regulate the fate of RNA polymerase II transcripts via mutually exclusive interactions with RNA effectors. One such effector is PHAX, which mediates the nuclear export of U-rich small nuclear RNAs (snRNAs). Here we present the cryo-electron microscopy structure of the human snRNA export complex comprising phosphorylated PHAX, CBC, CRM1-RanGTP and capped RNA. The central region of PHAX bridges CBC to the export factor CRM1-RanGTP, while also reinforcing cap dinucleotide binding. Additionally, PHAX interacts with a distant region of CRM1, facilitating contacts of the essential phosphorylated region of PHAX with the prominent basic surface of RanGTP. CBC engagement within the snRNA export complex is incompatible with its binding to other RNA effectors such as ALYREF or NCBP3. We demonstrate that snRNA export complex formation requires synergistic binding of all its components, which in turn displaces ARS2 from CBC and commits the complex for export.
- Université Grenoble Alpes, CNRS, CEA, IBS, Grenoble, France.
Organizational Affiliation: