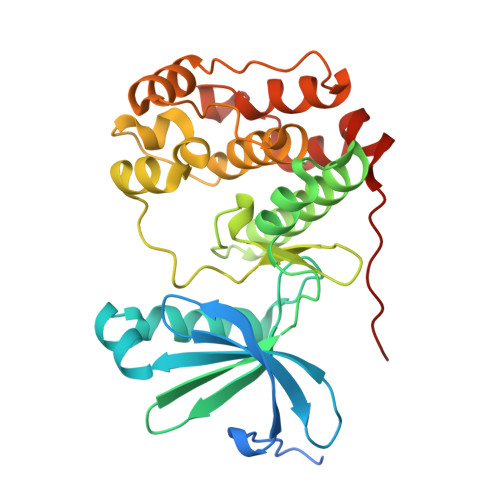
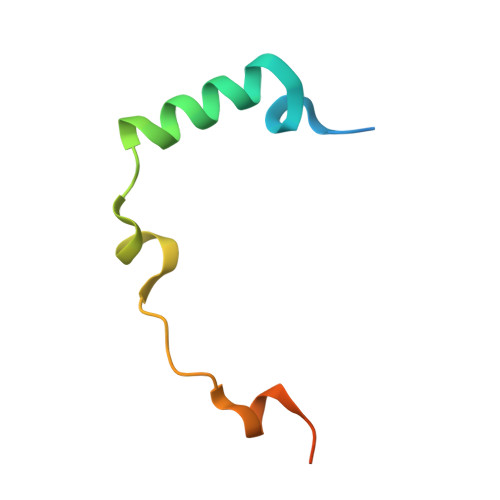
Surface-mutagenesis strategies to enable structural biology crystallization platforms.
Schaefer, M., Putter, V., Hilpmann, A., Egner, U., Holton, S.J., Hillig, R.C.(2024) Acta Crystallogr D Struct Biol 80: 661-674
- PubMed: 39207897
- DOI: https://doi.org/10.1107/S2059798324007939
- Primary Citation of Related Structures:
9ESA - PubMed Abstract:
A key prerequisite for the successful application of protein crystallography in drug discovery is to establish a robust crystallization system for a new drug-target protein fast enough to deliver crystal structures when the first inhibitors have been identified in the hit-finding campaign or, at the latest, in the subsequent hit-to-lead process. The first crucial step towards generating well folded proteins with a high likelihood of crystallizing is the identification of suitable truncation variants of the target protein. In some cases an optimal length variant alone is not sufficient to support crystallization and additional surface mutations need to be introduced to obtain suitable crystals. In this contribution, four case studies are presented in which rationally designed surface modifications were key to establishing crystallization conditions for the target proteins (the protein kinases Aurora-C, IRAK4 and BUB1, and the KRAS-SOS1 complex). The design process which led to well diffracting crystals is described and the crystal packing is analysed to understand retrospectively how the specific surface mutations promoted successful crystallization. The presented design approaches are routinely used in our team to support the establishment of robust crystallization systems which enable structure-guided inhibitor optimization for hit-to-lead and lead-optimization projects in pharmaceutical research.
- Structural Biology, Nuvisan ICB GmbH, Muellerstrasse 178, 13353 Berlin, Germany.
Organizational Affiliation: