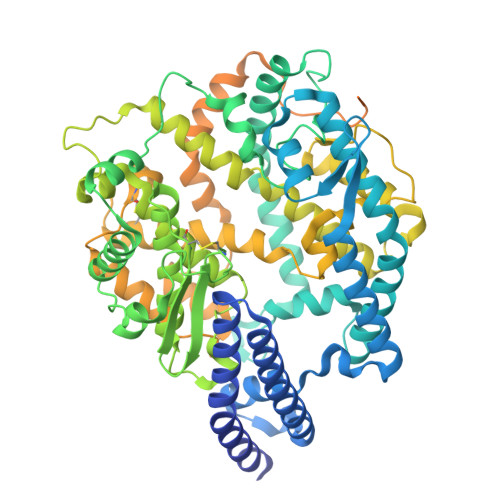
Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses.
Park, Y.J., Liu, C., Lee, J., Brown, J.T., Ma, C.B., Liu, P., Gen, R., Xiong, Q., Zepeda, S.K., Stewart, C., Addetia, A., Craig, C.J., Tortorici, M.A., Alshukairi, A.N., Starr, T.N., Yan, H., Veesler, D.(2025) Cell 188: 1711-1728.e21
- PubMed: 39922192
- DOI: https://doi.org/10.1016/j.cell.2024.12.032
- Primary Citation of Related Structures:
9D32, 9E0I, 9EA0, 9EH8 - PubMed Abstract:
DPP4 was considered a canonical receptor for merbecoviruses until the recent discovery of African bat-borne MERS-related coronaviruses using ACE2. The extent and diversity of ACE2 utilization among merbecoviruses and their receptor species tropism remain unknown. Here, we reveal that HKU5 enters host cells utilizing Pipistrellus abramus (P.abr) and several non-bat mammalian ACE2s through a binding mode distinct from that of any other known ACE2-using coronaviruses. We defined the molecular determinants of receptor species tropism and identified a single amino acid mutation enabling HKU5 to utilize human ACE2, providing proof of principle for machine-learning-assisted outbreak preparedness. We show that MERS-CoV and HKU5 have markedly distinct antigenicity and identified several HKU5 inhibitors, including two clinical compounds. Our findings profoundly alter our understanding of coronavirus evolution, as several merbecovirus clades independently evolved ACE2 utilization, and pave the way for developing countermeasures against viruses poised for human emergence.
- Department of Biochemistry, University of Washington, Seattle, WA 98195, USA; Howard Hughes Medical Institute, University of Washington, Seattle, WA 98195, USA.
Organizational Affiliation: