Membrane-bound OlyA (E69A)/PlyB (Hinge-Lock) prepore
Smothers, J., Chen, Z., Li, Y., Han, Y., Radhakrishnan, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ostreolysin A6 | A, B, D [auth L], E [auth M] | 143 | Pleurotus ostreatus | Mutation(s): 1 Gene Names: OlyA6 | 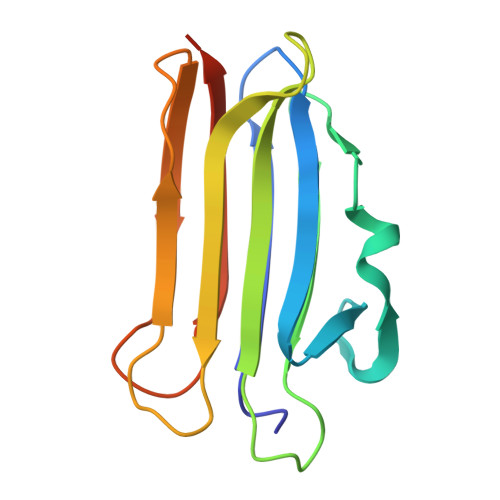 |
UniProt | |||||
Find proteins for P83467 (Pleurotus ostreatus) Explore P83467 Go to UniProtKB: P83467 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P83467 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Pleurotolysin B | C, F [auth N] | 483 | Pleurotus ostreatus | Mutation(s): 0 Gene Names: plyB | 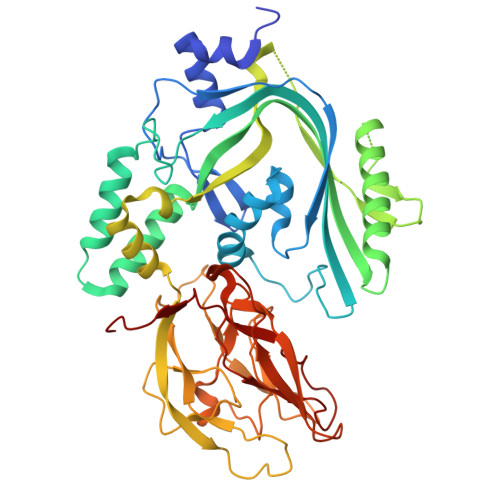 |
UniProt | |||||
Find proteins for Q5W9E8 (Pleurotus ostreatus) Explore Q5W9E8 Go to UniProtKB: Q5W9E8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5W9E8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A1A8R (Subject of Investigation/LOI) Query on A1A8R | G [auth A] H [auth A] I [auth A] J [auth A] K [auth B] | N-oleoyl-D-erythro-sphingosylphosphorylcholine C41 H82 N2 O6 P NBEADXWAAWCCDG-QDDWGVBQSA-O |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) | United States | HL160487 |
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | AI158357 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | T32GM131963 |
| Welch Foundation | United States | I-1793 |
| Leducq Foundation | France | 19CVD04 |