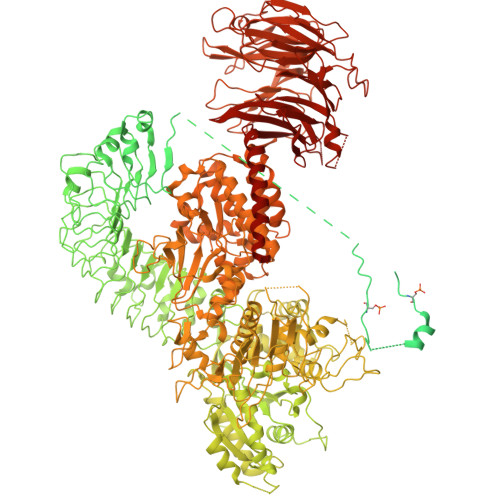
14-3-3 binding maintains the Parkinson's associated kinase LRRK2 in an inactive state.
Martinez Fiesco, J.A., Beilina, A., Alvarez de la Cruz, A., Li, N., Metcalfe, R.D., Cookson, M.R., Zhang, P.(2025) Nat Commun 16: 7226-7226
- PubMed: 40764514
- DOI: https://doi.org/10.1038/s41467-025-62337-1
- Primary Citation of Related Structures:
9CI3 - PubMed Abstract:
Leucine-rich repeat kinase 2 (LRRK2) is an essential regulator in cellular signaling and a major contributor to Parkinson's disease (PD) pathogenesis. 14-3-3 proteins are critical modulators of LRRK2 activity, yet the structural basis of their interaction has remained unclear. Here, we present the cryo-electron microscopy structure of the LRRK2:14-3-3 2 autoinhibitory complex, revealing how a 14-3-3 dimer stabilizes an autoinhibited LRRK2 monomer through dual-site anchoring. The dimer engages both phosphorylated S910/S935 sites and the COR-A/B subdomains within the Roc-COR GTPase region. This spatial configuration constrains LRR domain mobility, reinforces the inactive conformation, and likely impedes LRRK2 dimerization and oligomer formation. Structure-guided mutagenesis studies show that PD-associated mutations at the COR:14-3-3 2 interface and within the GTPase domain weaken 14-3-3 binding and impair its inhibitory effect on LRRK2 kinase activity. Furthermore, we demonstrate that type I LRRK2 kinase inhibitor, which stabilizes the kinase domain in its active conformation, reduces 14-3-3 binding and promotes dephosphorylation at pS910 and pS935. Together, these findings provide a structural basis for understanding how LRRK2 is maintained in an inactive state, elucidate the mechanistic role of 14-3-3 in LRRK2 regulation, inform the interpretation of PD biomarkers, and suggest therapeutic strategies aimed at enhancing LRRK2-14-3-3 interactions to treat PD and related disorders.
- Kinase Complexes Section, Center for Structural Biology, Center for Cancer Research, National Cancer Institute, Frederick, MD, USA.
Organizational Affiliation: