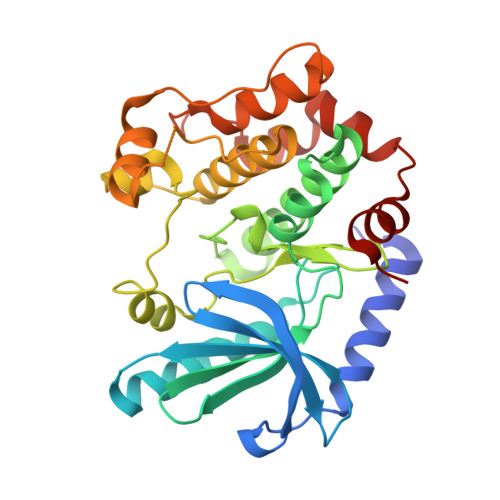
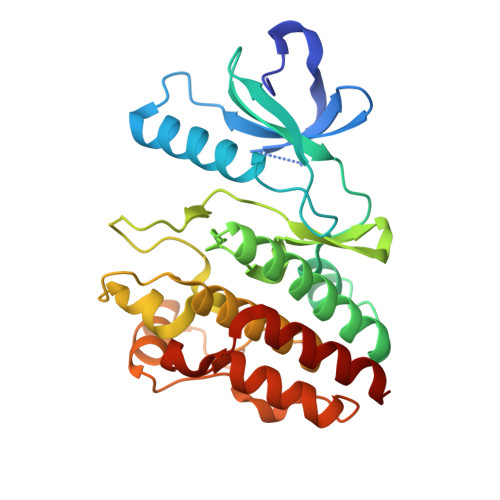
The Pan-RAF-MEK Nondegrading Molecular Glue NST-628 Is a Potent and Brain-Penetrant Inhibitor of the RAS-MAPK Pathway with Activity across Diverse RAS- and RAF-Driven Cancers.
Ryan, M.B., Quade, B., Schenk, N., Fang, Z., Zingg, M., Cohen, S.E., Swalm, B.M., Li, C., Ozen, A., Ye, C., Ritorto, M.S., Huang, X., Dar, A.C., Han, Y., Hoeflich, K.P., Hale, M., Hagel, M.(2024) Cancer Discov 14: 1190-1205
- PubMed: 38588399
- DOI: https://doi.org/10.1158/2159-8290.CD-24-0139
- Primary Citation of Related Structures:
9AXA, 9AXC, 9AXH, 9AXM, 9AXX, 9AXY, 9AY7, 9AYA - PubMed Abstract:
Alterations in the RAS-MAPK signaling cascade are common across multiple solid tumor types and is a driver for many cancers. NST-628 is a potent pan-RAF-MEK molecular glue that prevents phosphorylation and activation of MEK by RAF, overcoming the limitations of traditional RAS-MAPK inhibitors and leading to deep durable inhibition of the pathway. Cellular, biochemical, and structural analysis of RAF-MEK complexes show that NST-628 engages all isoforms of RAFand prevents the formation of BRAF-CRAF heterodimers, a differentiated mechanism from all current RAF inhibitors. With a potent and durable inhibition of the RAF-MEK signaling complex as well as high intrinsic permeability into the brain, NST-628 demonstrates broad efficacy in cellular and patient-derived tumor models harboring diverse MAPK pathway alterations, including orthotopic intracranial models. Given its functional and pharmacokinetic mechanisms that are differentiated from previous therapies , NST-628 is positioned to make an impact clinically in an areas of unmet patient need.
- Nested Therapeutics, Cambridge, MA, United States.
Organizational Affiliation: