The molecular basis of mu-opioid receptor signaling plasticity.
Zhang, H., Wang, X., Xi, K., Shen, Q., Xue, J., Zhu, Y., Zang, S.K., Yu, T., Shen, D.D., Guo, J., Chen, L.N., Ji, S.Y., Qin, J., Dong, Y., Zhao, M., Yang, M., Wu, H., Yang, G., Zhang, Y.(2025) Cell Res 35: 1021-1036
- PubMed: 41199005
- DOI: https://doi.org/10.1038/s41422-025-01191-8
- Primary Citation of Related Structures:
9WST, 9WSV, 9WSW, 9WSX - PubMed Abstract:
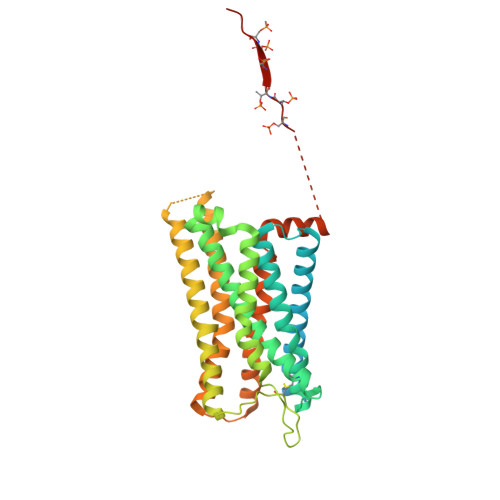
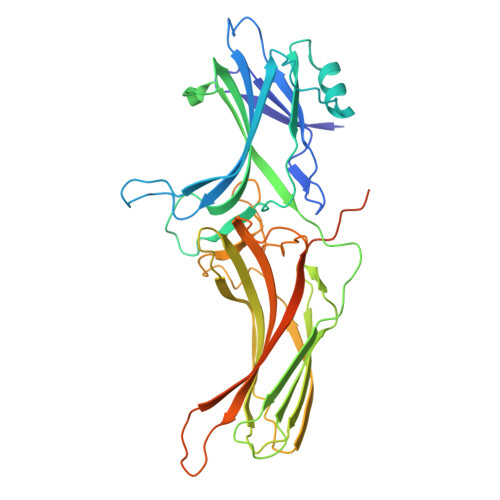
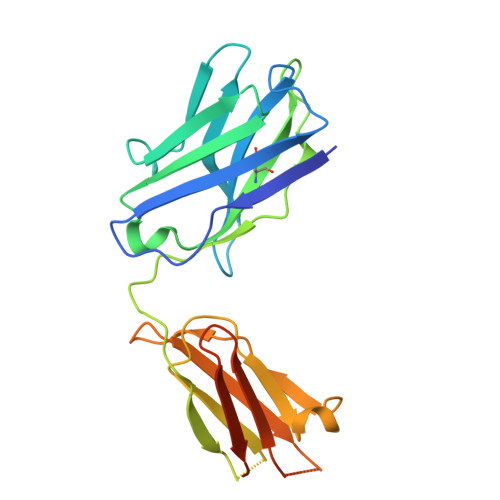
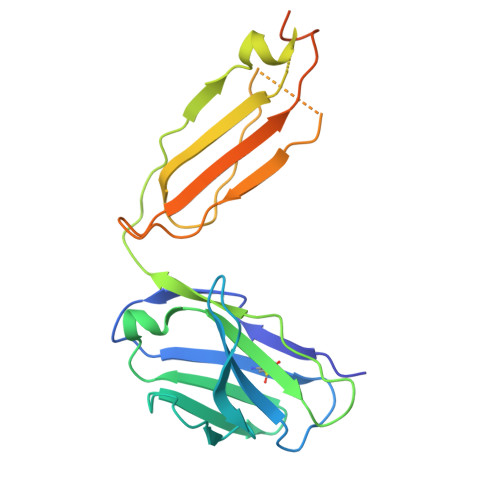
Activation of the μ-opioid receptor (μOR) alleviates pain but also elicits adverse effects through diverse G proteins and β-arrestins. The structural details of μOR complexes with G z and β-arrestins have not been determined, impeding a comprehensive understanding of μOR signaling plasticity. Here, we present the cryo-EM structures of the μOR-G z and μOR-βarr1 complexes, revealing selective conformational preferences of μOR when engaged with specific downstream signaling transducers. Integrated receptor pharmacology, including high-resolution structural analysis, cell signaling assays, and molecular dynamics simulations, demonstrated that transmembrane helix 1 (TM1) acts as an allosteric regulator of μOR signaling bias through differential stabilization of the G i -, G z -, and βarr1-bound states. Mechanistically, outward TM1 displacement confers structural flexibility that promotes G protein recruitment, whereas inward TM1 retraction facilitates βarr1 recruitment by stabilizing the intracellular binding pocket through coordinated interactions with TM2, TM7, and helix8. Structural comparisons between the G i -, G z -, and βarr1-bound complexes identified a TM1-fusion pocket with significant implications for downstream signaling regulation. Overall, we demonstrate that the conformational and thermodynamic heterogeneity of TM1 allosterically drives the downstream signaling specificity and plasticity of μOR, thereby expanding the understanding of μOR signal transduction mechanisms and providing new avenues for the rational design of analgesics.
- Department of Pathology of Sir Run Run Shaw Hospital, Department of Pharmacology, MOE Frontier Science Center for Brain Research and Brain-Machine Integration, and Liangzhu Laboratory, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Organizational Affiliation: