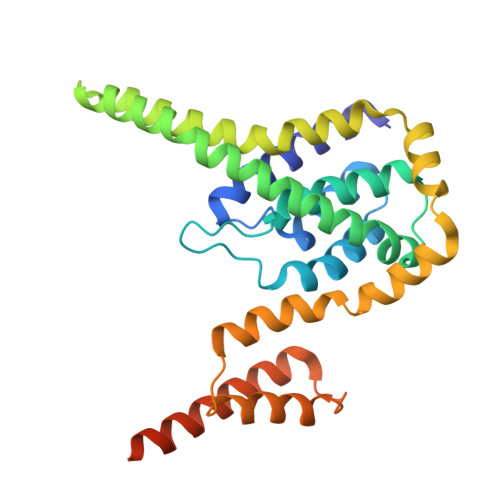
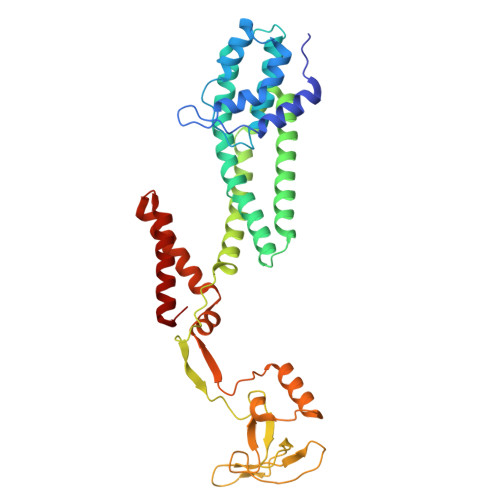
Structural basis for the extended-spectrum antimicrobial activity of Garvieacin Q.
Duan, J., Li, D., Zhao, Y., Wang, J.(2026) Appl Environ Microbiol : e0177325-e0177325
- PubMed: 41562606
- DOI: https://doi.org/10.1128/aem.01773-25
- Primary Citation of Related Structures:
9WJR, 9WJU, 9WJW - PubMed Abstract:
Class IIa and IId bacteriocins are antimicrobial peptides with potential for combating antibiotic-resistant pathogens. However, their species-specific activity, dictated by recognition of the mannose phosphotransferase system (Man-PTS) receptor, often restricts their spectrum. Garvieacin Q/Garvicin Q (GarQ), a newly identified class IId bacteriocin, is unusual in that it targets both Lactococcus garvieae and the non-lactococcal pathogen Listeria monocytogenes , yet the structural basis for this cross-species activity has remained unclear. Using cryo-electron microscopy, we determined the structures of GarQ bound to Man-PTS receptors from Lactococcus garvieae and Listeria monocytogenes . In Lactococcus garvieae , the receptor contains a unique Tudor-like γ+ domain that sterically constrains the N terminus of incoming bacteriocins, thereby enforcing specificity for GarQ while excluding others such as lactococcin A (LcnA). In Listeria monocytogenes , GarQ engages the receptor through the same conserved binding mode, effectively bypassing the unusual species barrier. We further demonstrate that the C-terminal length of GarQ is a critical determinant of pore size and target specificity. Together, these findings uncover the structural mechanism underlying GarQ's atypical extended-spectrum activity and provide a framework for engineering bacteriocins with customized spectra to combat specific pathogens.IMPORTANCEThis study establishes a structural basis for how the extended-spectrum bacteriocin Garvieacin Q (GarQ) circumvents the canonical species-specificity of class II bacteriocins by engaging mannose phosphotransferase system receptors from different bacterial genera through both conserved and divergent binding modes. We identify a previously unknown Tudor-like γ+ domain in the Lactococcus garvieae receptor that sterically restricts the access of other bacteriocins, thereby defining bacteriocin specificity. Moreover, we demonstrate that the C-terminal length of GarQ critically determines pore size and bacterial targets, revealing an engineerable principle for designing synthetic bacteriocins with customized spectra against clinically relevant pathogens.
- State Key Laboratory of Membrane Biology, Beijing Frontier Research Center for Biological Structure, School of Life Sciences, Tsinghua University, Beijing, People's Republic of China.
Organizational Affiliation: