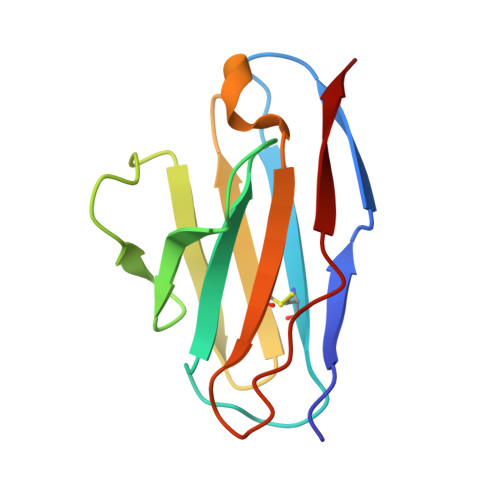
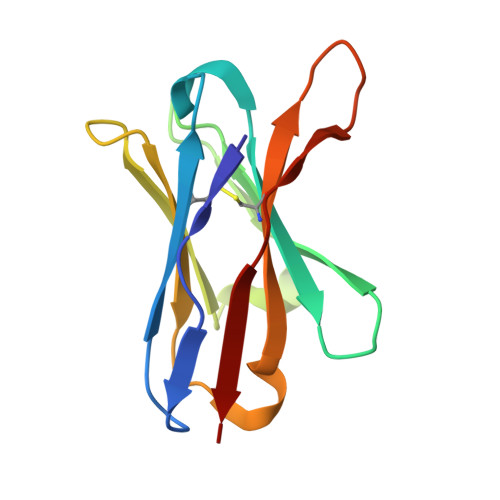
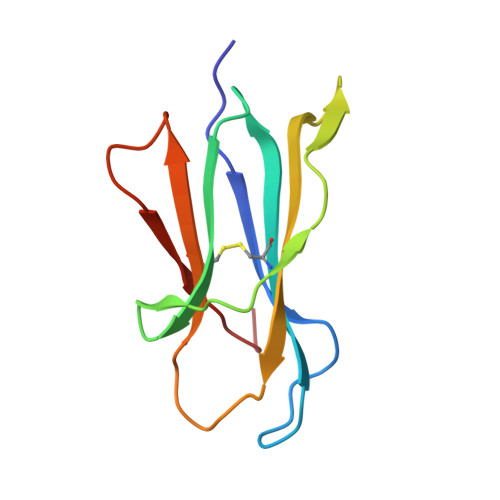
Crystal structure of the beta 2 -microglobulin-BBM.1 antibody complex reveals the molecular basis of antigen recognition.
Wu, J., Zeng, F., Wang, X., Wei, P.(2025) Acta Crystallogr D Struct Biol 81: 473-481
- PubMed: 40748259
- DOI: https://doi.org/10.1107/S2059798325006370
- Primary Citation of Related Structures:
9UVI - PubMed Abstract:
β 2 -Microglobulin (β 2 M) is an essential component of major histocompatibility complex class I (MHC-I) molecules, with a well established canonical role in immune surveillance. Beyond its classical functions, accumulating evidence has highlighted β 2 M as a multifaceted biomarker, with elevated serum levels closely associated with disease burden and prognosis in metabolic disorders, malignancies, autoimmune diseases and central nervous system conditions. In this study, we resolved the crystal structure of human β 2 M in complex with the mouse monoclonal antibody BBM.1 at 2.50 Å resolution using X-ray crystallography. Structural analysis revealed that BBM.1 binds β 2 M through multiple CDRs, recognizing key surface residues including Glu36, Asp38, Lys41, Asn42, Glu44, Arg45, Glu47 and Arg81. The interaction is anchored by a central hydrophobic core formed by Trp32 (light chain), Trp99 (heavy chain) and Ile92 (β 2 M), which is deeply buried in the interface. Surrounding this core is a well organized polar interaction network composed of hydrogen bonds and salt bridges, primarily involving β 2 M residues Lys41, Glu44, Arg45 and Glu47. Notably, the Arg45 residue deeply embeds into the antibody-binding pocket, forming several crucial interactions. These findings not only validate previous biochemical and mutational data but also identify new epitope residues, providing a structural foundation for the development and optimization of precision therapeutic strategies targeting β 2 M.
- Guangxi Key Laboratory of Special Biomedicine, School of Medicine, Guangxi University, Nanning 530004, People's Republic of China.
Organizational Affiliation: