Structure of the complex of LGR4_ECD with Norrin
Geng, Y., Hu, F.Z., Qiao, H.R.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MB52 | 556 | Camelus ferus | Mutation(s): 0 | 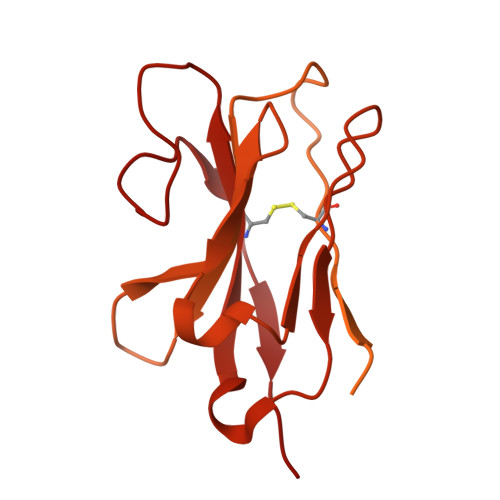 | |
UniProt | |||||
Find proteins for B5Z8H1 (Helicobacter pylori (strain G27)) Explore B5Z8H1 Go to UniProtKB: B5Z8H1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B5Z8H1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Leucine-rich repeat-containing G-protein coupled receptor 4 | B [auth C] | 954 | Homo sapiens | Mutation(s): 0 Gene Names: LGR4, GPR48 | 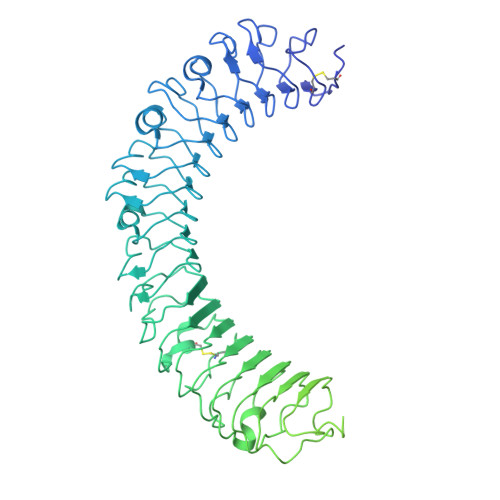 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9BXB1 (Homo sapiens) Explore Q9BXB1 Go to UniProtKB: Q9BXB1 | |||||
PHAROS: Q9BXB1 GTEx: ENSG00000205213 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9BXB1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Norrin,Immunoglobulin gamma-1 heavy chain | C [auth E], D [auth F] | 409 | Homo sapiens | Mutation(s): 0 Gene Names: NDP, EVR2 | 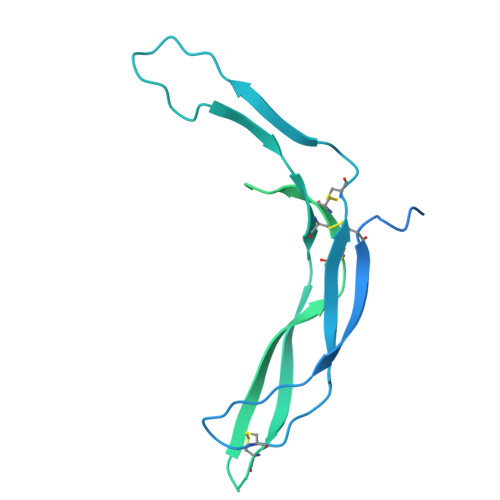 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q00604 (Homo sapiens) Explore Q00604 Go to UniProtKB: Q00604 | |||||
PHAROS: Q00604 GTEx: ENSG00000124479 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q00604 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | -- |