Discovery and molecular mechanism of potent neutralizing antibody from humanized mice with respiratory syncytial virus.
Zhang, Z., Feng, R., Zhang, L., Yang, Q., Chen, X., Wang, X., Nie, C., Peng, W., Wang, X., Zhu, L., Guo, Y., Sun, Z.(2025) PLoS Pathog 21: e1013674-e1013674
- PubMed: 41248206
- DOI: https://doi.org/10.1371/journal.ppat.1013674
- Primary Citation of Related Structures:
9U74 - PubMed Abstract:
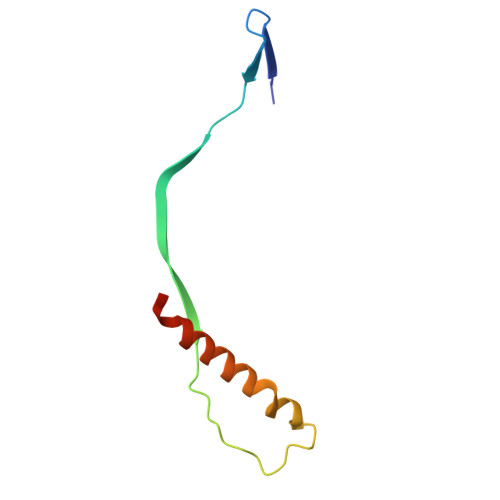
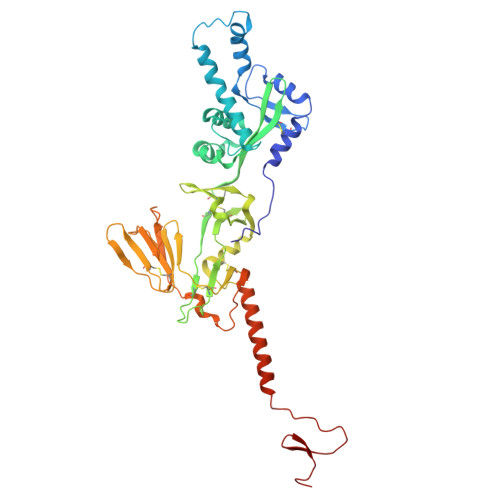
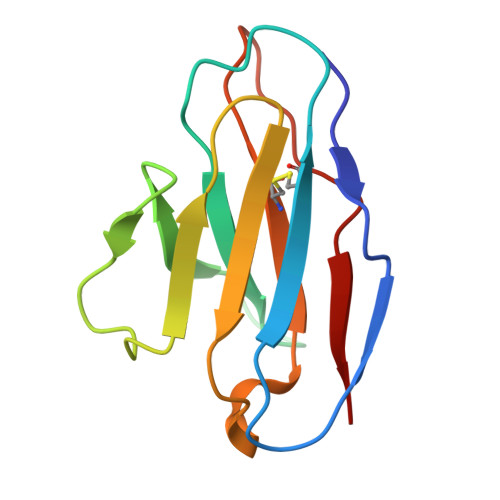
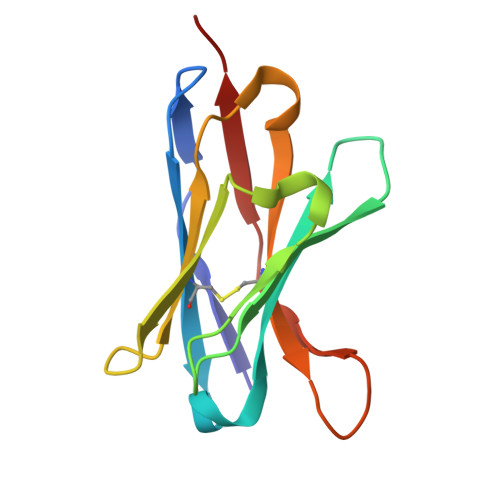
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory tract infections among infants and older adults, posing a significant threat to global public health. The prophylactic use of neutralizing antibodies (nAbs) underscores the need to understand elite RSV antibody neutralization mechanisms, which is fundamental for developing next-generation therapies with enhanced potency and broader activity. In this study, we utilized H2L2 transgenic mice encoding human immunoglobulin variable regions for immunization and successfully screened multiple antibodies with significant neutralizing activity using the Beacon Optofluidic system. One of these antibodies, PR306007, exhibited significantly superior broad-spectrum neutralization against both RSV-A and B subgroups. Cryo-electron microscopy (Cryo-EM) structural analysis revealed that PR306007 binds to a unique epitope that overlaps with antigenic sites II and V of the F protein, with its primary binding regions located at the base of the α6 and α7 helices of site II, and residues S173 and N175 of site V. This binding mode offers valuable insights into enhanced neutralization activity and potentially reduces the risk of emerging immune evasive mutants. Furthermore, PR306007 showed potent in vivo antiviral activity against RSV infection and demonstrated good efficacy against both lower and upper respiratory tract infections, making it a promising prophylactic candidate for broad prevention. These findings provide new insights for the future development of RSV vaccines or nAbs.
- Graduate School of Guangzhou Medical University, Panyu District, Guangzhou, China.
Organizational Affiliation: