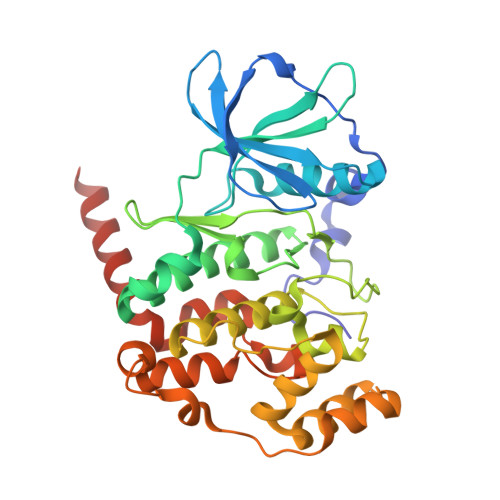
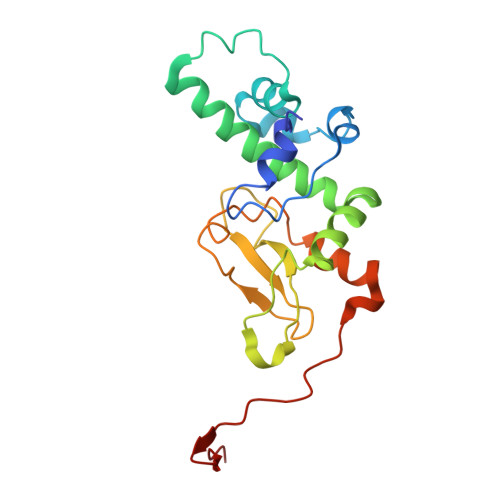
An inositol pyrophosphate interaction screen provides insight into the regulation of plant casein kinase II.
Sturm, K., Pri-Tal, O., Rico-Resendiz, F., Verma, Y., Richter, A., Chen, H., Broger, L., Hothorn, L.A., Fiedler, D., Panse, V.G., Hothorn, M.To be published.