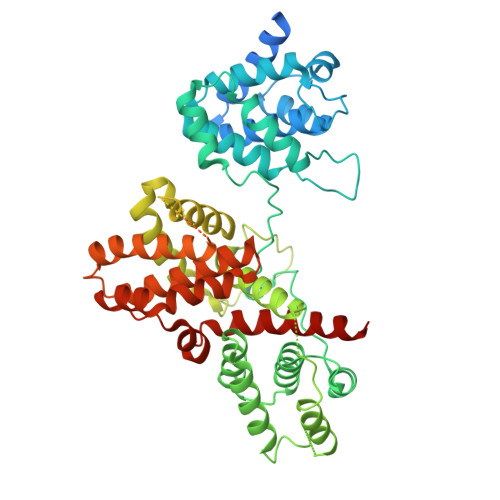
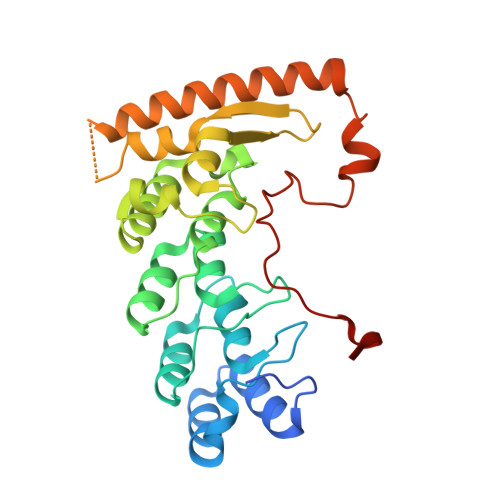
Crystal structure of the Legionella pneumophila effector SidL (Lpg0437) in complex with its metaeffector LegA11 (Lpg0436).
Machtens, D.A., Hutchison, C.A., Stein, A.M., Eberhage, J., Willerding, J.M., Eschenburg, S., Shames, S.R., Reubold, T.F.(2025) bioRxiv
- PubMed: 41278709
- DOI: https://doi.org/10.1101/2025.10.23.683979
- Primary Citation of Related Structures:
9SXC - PubMed Abstract:
Legionella pneumophila is an opportunistic human pathogen that causes atypical pneumonia called Legionnaires' Disease. To replicate within host cells, L. pneumophila injects up to 330 effector proteins into the host cytosol via a Dot/Icm type IV secretion system. Several effectors, called metaeffectors, regulate the activity of other effectors within infected host cells through direct protein-protein interactions. LegA11 (AnkJ/Lpg0436) has been identified as a putative metaeffector of SidL (Ceg14/Lpg0437), one of eight L. pneumophila effectors that inhibit host mRNA translation. LegA11 binds and suppresses SidL enzymatic activity, but the molecular basis of this regulation and impact on mRNA translation are unknown. Here, we present the crystal structure of SidL in complex with LegA11 to a resolution of 2.4 Å, revealing a high-affinity 1:1 complex with an extensive interaction interface of ~2300 Å 2 . Using isothermal titration calorimetry, we determined a dissociation constant of 1.8 nM. In vitro translation assays demonstrate that SidL inhibits mRNA translation, and this activity is completely suppressed by LegA11. Mutagenesis of key interface residues in LegA11 disrupts complex formation and abolishes its metaeffector activity, confirming that LegA11 regulates SidL through direct protein-protein interaction. These findings establish LegA11 as a bona fide metaeffector that contributes to suppression of host mRNA translation by L. pneumophila .
- Institute for Biophysical Chemistry, Hannover Medical School, Carl-Neuberg-Straße 1, 30625 Hannover, Germany.
Organizational Affiliation: