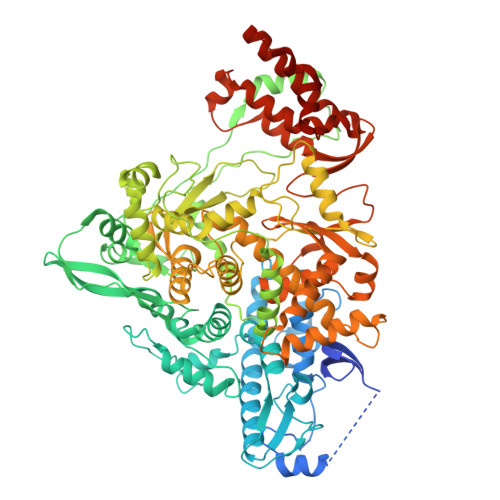
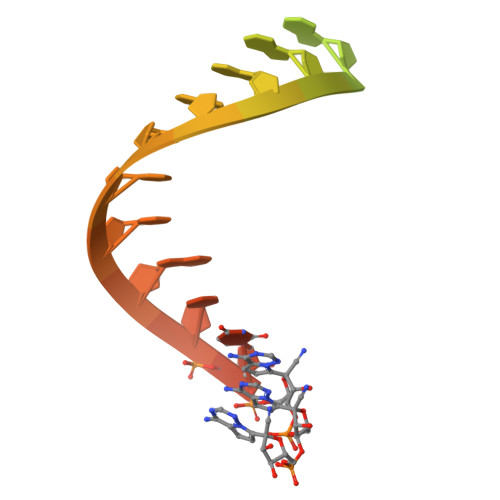
Structural basis for selective remdesivir incorporation by SARS-CoV-2 RNA polymerase, and S759A resistance.
Gordon, C.J., Oliva, M.F., Lee, H.W., Goovaerts, Q., De Wijngaert, B., Gotte, M., Das, K.(2025) bioRxiv
- PubMed: 40950230
- DOI: https://doi.org/10.1101/2025.09.04.674295
- Primary Citation of Related Structures:
9SAO, 9SAP, 9SAQ, 9SAR - PubMed Abstract:
Nucleoside analogs (NAs) have been successfully used to treat viral infections. dNTP analogs are primarily DNA chain terminators, while NTP analogs, such as remdesivir, can inhibit as delayed chain terminators or when in the template strand. Determining the frequency of remdesivir triphosphate (RTP) incorporation in the presence of the competing ATP can help in understanding different modes of viral RNA-dependent RNA polymerase (RdRp) inhibition by NTP analogs. We employed enzymatic assays, mass spectrometry, and cryo-EM to show that SARS-CoV-2 RdRp preferentially incorporates RTP, outcompeting 10-fold higher ATP concentration; however, successive RTP incorporations are less favoured when ATP is present. Structures of SARS-CoV-2 RdRp in this and previous studies demonstrate resilience of remdesivir:UMP base pair to translocation, explaining the reduced preference for conjugate incorporations. Together, the RTP versus ATP incorporation is driven by their relative concentration and structural rigidity of remdesivir:UMP, ultimately limiting the number of incorporated remdesivir in a fully synthesized RNA strand. The S759A mutant confers RTP resistance, and the structures of S759A RdRp catalytic complexes reveal that altered ribose-ring conformation and repositioning of the primer 3'-end nucleotide contribute to RTP resistance. These findings enhance our understanding of non-obligate NTP analogs and provide insight into S759A resistance mechanism.