Enzyme-Activated Sugar-Coated Bifunctional Degraders.
Zhu, Q., Fischer, G., Cheng, S.S., Payne, N.C., Peter, D., Mody, A.C., Arce-Solano, S., Shen, D., Lin, Z., Mazitschek, R., Kessler, D., Woo, C.M.(2025) J Am Chem Soc 147: 34672-34680
- PubMed: 40937862
- DOI: https://doi.org/10.1021/jacs.5c09843
- Primary Citation of Related Structures:
9SAF, 9SAI - PubMed Abstract:
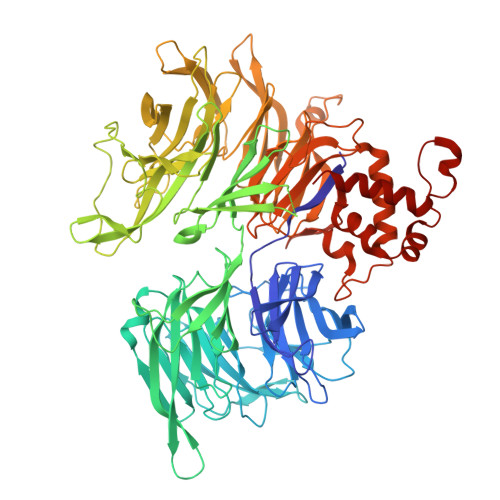
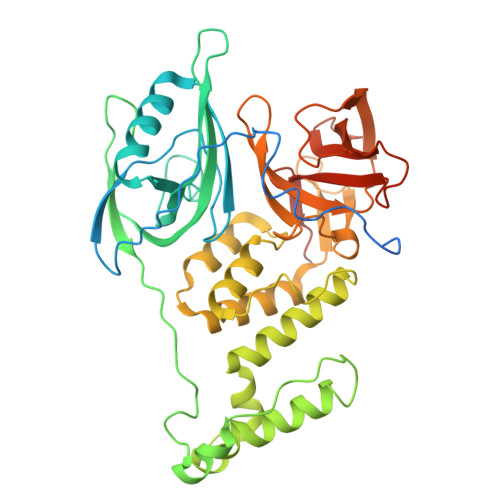
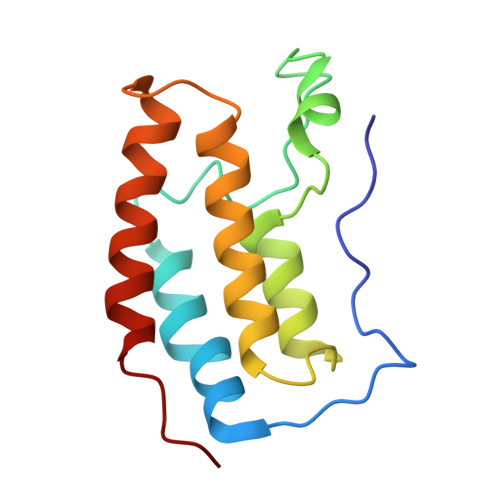
Targeted protein degradation with compounds like proteolysis targeting chimeras (PROTACs) directs disease-associated proteins to the E3 ligase ubiquitin-proteasome system for removal. However, commonly employed E3 ligases such as cereblon (CRBN) are broadly expressed. To metabolically gate PROTAC activity, we developed an enzymatic activation strategy by integrating an O-GlcNAc modification to the cyclimids, ligands derived from the natural motifs recognized by CRBN. These sugar-coated PROTACs (SCPs) were designed using structural analyses of representative cyclimid degraders complexed with CRBN and target protein BRD4. We found that glycosylation of the cyclimid reduced CRBN binding and complex formation with BRD4 until enzymatic removal of the O-GlcNAc moiety by O-GlcNAcase (OGA). The requirement for enzymatic activation is demonstrated by in vitro biochemical binding, cellular degradation, and cell viability assays in engineered and native cell lines. O-GlcNAc is thus an effective mechanism to gate targeted protein degradation modalities that motivates the development of similar strategies to enhance selectivity with other protein modifications.
- Department of Chemistry and Chemical Biology, Harvard University, Cambridge, Massachusetts 02138, United States.
Organizational Affiliation: