Crystal structure of mouse pVHL-ElonginB-ElonginC complex
Blat, A., Biterova, E., Cukier, C.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| von Hippel-Lindau disease tumor suppressor | 164 | Mus musculus | Mutation(s): 0 Gene Names: Vhl, Vhlh | 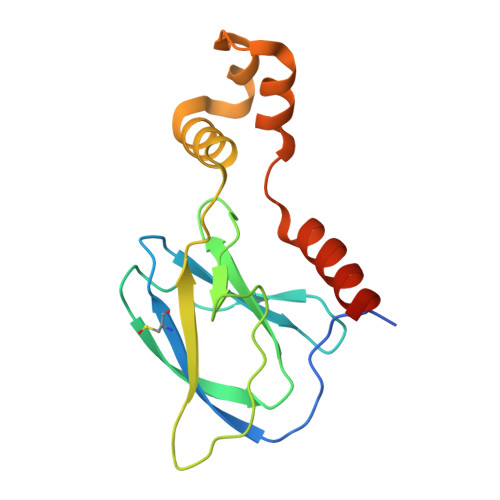 | |
UniProt | |||||
Find proteins for P40338 (Mus musculus) Explore P40338 Go to UniProtKB: P40338 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P40338 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Elongin-B | 104 | Mus musculus | Mutation(s): 0 Gene Names: Elob, Tceb2 | 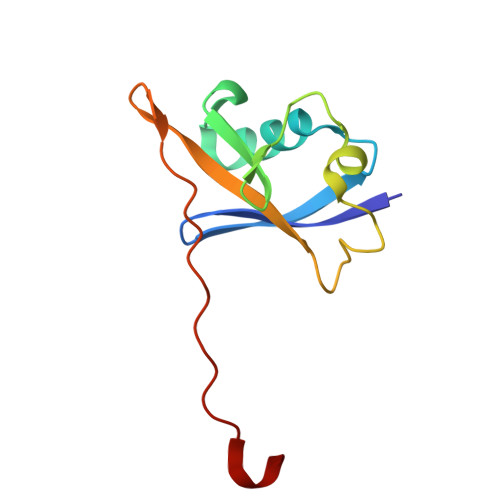 | |
UniProt | |||||
Find proteins for P62869 (Mus musculus) Explore P62869 Go to UniProtKB: P62869 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62869 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Elongin-C | 97 | Mus musculus | Mutation(s): 0 Gene Names: Eloc, Tceb1 | 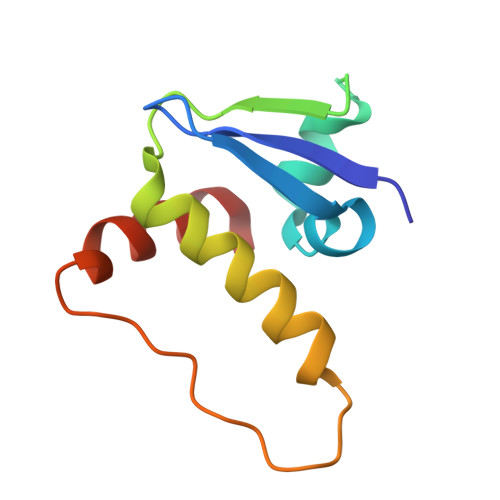 | |
UniProt | |||||
Find proteins for P83940 (Mus musculus) Explore P83940 Go to UniProtKB: P83940 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P83940 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PGE Query on PGE | D [auth A] | TRIETHYLENE GLYCOL C6 H14 O4 ZIBGPFATKBEMQZ-UHFFFAOYSA-N |  | ||
| IOD Query on IOD | E [auth A], G [auth B], I [auth C] | IODIDE ION I XMBWDFGMSWQBCA-UHFFFAOYSA-M |  | ||
| PEG Query on PEG | J [auth C] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| EDO Query on EDO | F [auth A], H [auth B], K [auth C], L [auth C] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSX Query on CSX | A | L-PEPTIDE LINKING | C3 H7 N O3 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 73.48 | α = 90 |
| b = 59.78 | β = 100.85 |
| c = 91.67 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Other private | Poland | -- |