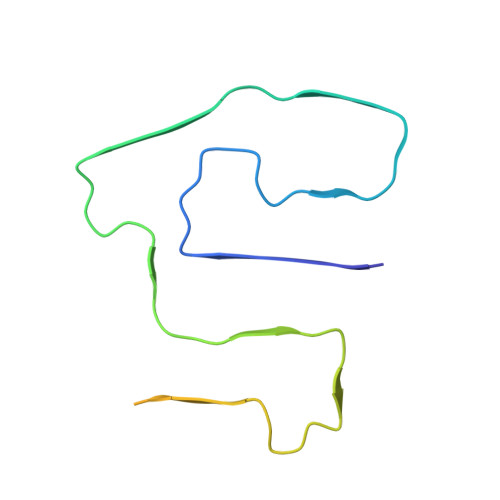
Cryo-EM Observation of AA Amyloid Fibrils in Mouse Model of Systemic AApoAII Amyloidosis.
Andreotti, G., Higuchi, K., Schmidt, M., Fandrich, M.(2025) J Mol Biology 437: 169438-169438
- PubMed: 40945578
- DOI: https://doi.org/10.1016/j.jmb.2025.169438
- Primary Citation of Related Structures:
9R4Z - PubMed Abstract:
The co-deposition of amyloid fibrils from different precursor proteins is a topic of increasing relevance for protein misfolding diseases. Using cryo-electron microscopy (cryo-EM), we here determined the structures of two serum amyloid A (SAA) protein-derived amyloid fibril morphologies that were extracted from a mouse strain that is primarily known to be associated with apolipoprotein A-II-derived amyloid fibrils. The two fibril morphologies show the same protomer conformation as in previously reported ex vivo amyloid fibrils from SAA protein but a different relative arrangement of fibril protein stacks. These data establish that serum amyloid A-derived amyloid fibrils share the same fibril protein fold in different mouse strains and disease contexts.
- Institute of Protein Biochemistry, Ulm University, 89081 Ulm, Germany. Electronic address: giada.andreotti@uni-ulm.de.
Organizational Affiliation: