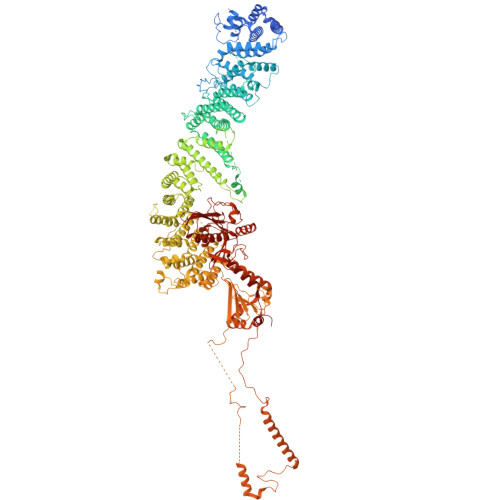
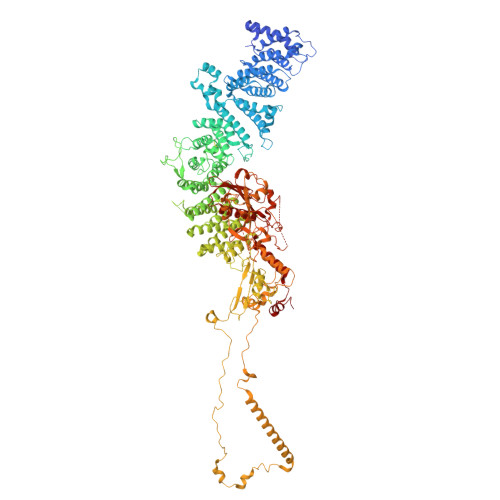
Structure and mechanism of the RalGAP tumor suppressor complex.
Rasche, R., Klink, B.U., Apken, L.H., Michalke, E., Chen, M., Oeckinghaus, A., Gatsogiannis, C., Kummel, D.(2025) Nat Commun 16: 7002-7002
- PubMed: 40738882
- DOI: https://doi.org/10.1038/s41467-025-61743-9
- Primary Citation of Related Structures:
9QWP - PubMed Abstract:
The RalGAP (GTPase activating protein) complexes are negative regulators of the Ral GTPases and thus crucial components that counteract oncogenic Ras signaling. However, no structural information on the architecture of this tumor suppressor complex is available hampering a mechanistic understanding of its functionality. Here, we present a cryo-EM structure of RalGAP that reveals an extended 58 nm tetrameric architecture comprising two heterodimers of the RalGAPα and RalGAPβ subunits. We show that the catalytic domain of RalGAPα requires stabilization by a unique domain of RalGAPβ, providing the molecular basis for why RalGAP complexes are obligatory heterodimers. Formation of RalGAP tetramers is not required for activity in vitro, but essential for function of the complex in vivo. Structural analysis of RalGAP subunit variants reported in cancer patients suggests effects on complex formation and thus functional relevance, emphasizing the significance of the obtained structural information for medical research.
- Institute of Biochemistry, University of Münster, Münster, Germany.
Organizational Affiliation: