Distal peptide elongation by a protease-like ligase and two distinct carrier proteins
Gude, F., Bohne, A., Dell, M., Franke, J., Dunbar, K.L., Groll, M., Hertweck, C.(2025) Chem : 102740
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2025) Chem : 102740
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Butirosin biosynthesis protein H N-terminal domain-containing protein | 315 | Ruminiclostridium cellulolyticum | Mutation(s): 1 Gene Names: Ccel_3254 | 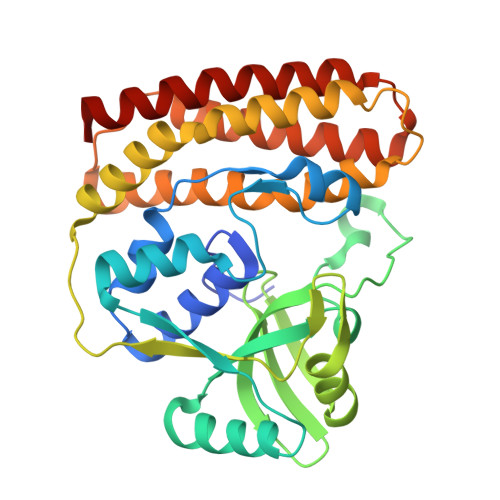 | |
UniProt | |||||
Find proteins for B8I0Y7 (Ruminiclostridium cellulolyticum (strain ATCC 35319 / DSM 5812 / JCM 6584 / H10)) Explore B8I0Y7 Go to UniProtKB: B8I0Y7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B8I0Y7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NA Query on NA | B [auth A] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 46.61 | α = 90 |
| b = 60.28 | β = 108.76 |
| c = 54.84 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| XFIT | data reduction |
| pointless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| German Research Foundation (DFG) | Germany | -- |