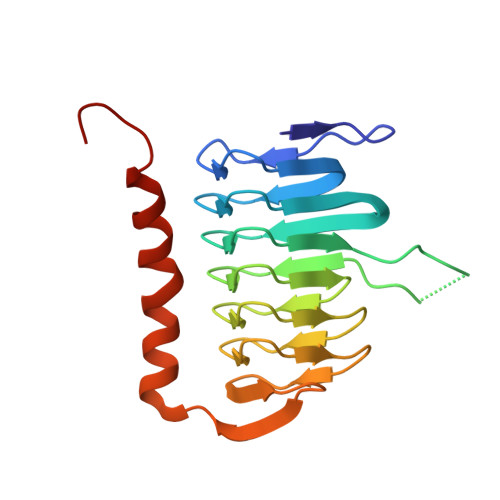
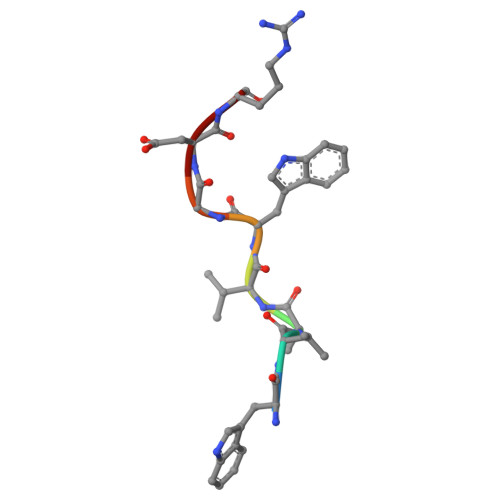
Structural and functional characterization of a metagenomically derived gamma-type carbonic anhydrase and its engineering into a hyperthermostable esterase.
Bodourian, C.S., Imran, M., Georgakis, N.D., Papageorgiou, A.C., Labrou, N.E.(2025) Protein Sci 34: e70396-e70396
- PubMed: 41294346
- DOI: https://doi.org/10.1002/pro.70396
- Primary Citation of Related Structures:
9QEV, 9QEZ - PubMed Abstract:
The 16S microbial community profiling of a metagenomics library from geothermal spring at Lisvori (Lesvos island, Greece) enabled the identification of a putative sequence exhibiting 95% identity to the γ-type carbonic anhydrase (γ-CA) from Caloramator australicus (γ-CaCA). The sequence of γ-CaCA was amplified by PCR, cloned, and expressed in E. coli. Activity assays showed that γ-CaCA possesses very low, but detectable, anhydrase activity, while exhibiting no measurable esterase activity. Differential scanning fluorimetry (DSF) revealed that the enzyme shows high thermal stability with a melting temperature (T m ) approximately 65-75°C in the pH range between 5.5 and 9.0. The structure of γ-CaCA was determined by X-ray crystallography at 1.11 Å resolution, the highest resolution reported so far for a γ-CA. The enzyme was crystallized as a trimer in the crystallographic asymmetric unit and contains three zinc-binding sites, one at each interface of neighboring subunits of the trimer. Structure-based rational design enabled the design and creation of a mutant enzyme (γ-CaCAmut) which possessed a heptapeptide insertion at the active-site loop and two-point mutations. Kinetic analysis demonstrated that γ-CaCAmut was successfully converted into a catalytically active esterase indicating successful activity gain through structure-guided engineering. The thermostability of γ-CaCAmut was significantly increased, aligning with the thermostability typically observed in hyperthermostable enzymes. X-ray crystallographic analysis of the γ-CaCAmut structure at 2.1 Å resolution, provided detailed structural insights into how the mutations impact the overall enzyme structure, function, and thermostability. These findings provide valuable structural and functional insights into γ-CAs and demonstrate a strategy for converting an inactive enzyme into a catalytically active form through rational design.
- Laboratory of Enzyme Technology, Department of Biotechnology, School of Applied Biology and Biotechnology, Agricultural University of Athens, Athens, Greece.
Organizational Affiliation: