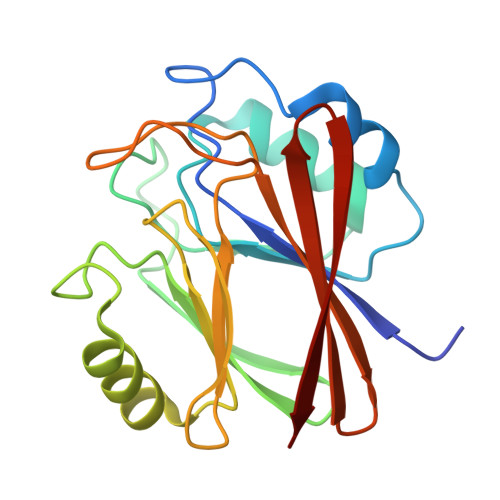
Mapping of endosomal proximity proteomes reveals Retromer as a hub for RAB GTPase regulation.
Anton-Plagaro, C., Chen, K.E., Guo, Q., Liu, M., Evans, A.J., Lewis, P.A., Heesom, K.J., Wilkinson, K.A., Collins, B.M., Cullen, P.J.(2025) Nat Commun 16: 6990-6990
- PubMed: 40738907
- DOI: https://doi.org/10.1038/s41467-025-61802-1
- Primary Citation of Related Structures:
9O9I - PubMed Abstract:
Endosomal retrieval and recycling of integral cargo proteins is essential for cell and organism development and homeostasis and is orchestrated through a specialised endosomal nanodomain, the retrieval sub-domain. Sub-domain dysfunction is associated with human disease, but our mechanistic understanding of its function remains poorly described. Here, using proximity proteomics of retrieval sub-domain components Retromer and Retriever we identify mechanistic details of retrieval sub-domain composition and organization, including an unrecognised complexity in the interface with RAB GTPase switching. Combining X-ray crystallography and in silico predictions with biochemical and cellular analysis, we reveal that Retromer directly associates and recruits the RAB10 regulators DENND4A, DENND4C, TBC1D1, and TBC1D4, and the RAB35 regulator TBC1D13 to regulate retrieval sub-domain function. The retrieval sub-domain therefore constitutes a hub for integrating cargo recycling with the regulated switching of selected RAB GTPases. We propose this constitutes a major component of the neuroprotective role of the retrieval sub-domain.
- School of Biochemistry, Faculty of Life Sciences, Biomedical Sciences Building, University of Bristol, Bristol, UK. carlos.antonplagaro@bristol.ac.uk.
Organizational Affiliation: