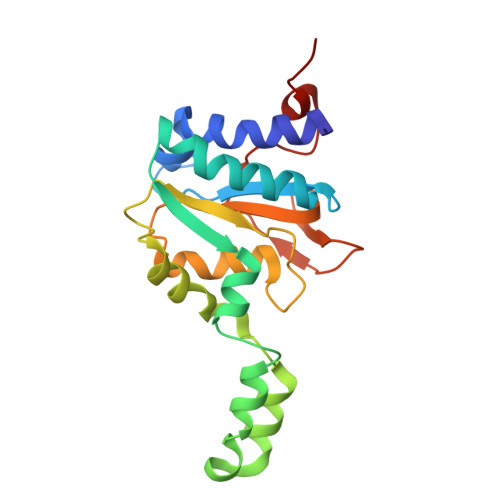
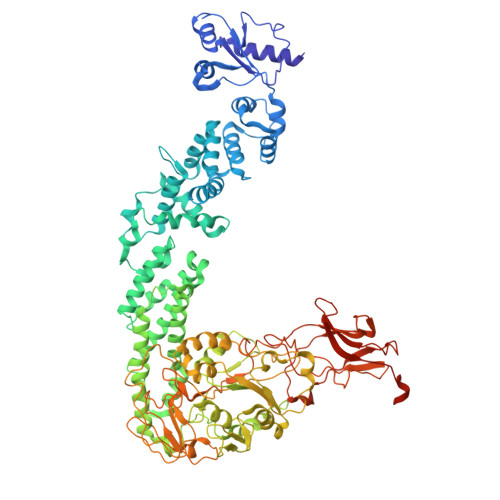
PglZ from Type I BREX phage defence systems is a metal-dependent nuclease that forms a sub-complex with BrxB.
Readshaw, J.J., Doyle, L.A., Puiu, M., Kelly, A., Nelson, A., Kaiser, A.J., McGuire, S.F., Peralta Acosta, J., Smith, D.L., Stoddard, B.L., Kaiser, B.K., Blower, T.R.(2025) Nucleic Acids Res 53
- PubMed: 40548935
- DOI: https://doi.org/10.1093/nar/gkaf540
- Primary Citation of Related Structures:
9NV3 - PubMed Abstract:
BREX (Bacteriophage Exclusion) systems, identified through shared identity with Pgl (Phage Growth Limitation) systems, are a widespread, highly diverse group of phage defence systems found throughout bacteria and archaea. The varied BREX Types harbour multiple protein subunits (between four and eight) and all encode a conserved putative phosphatase, PglZ, and an equally conserved, putative ATPase, BrxC. Almost all BREX systems also contain a site-specific methyltransferase, PglX. Despite having determined the structure and fundamental biophysical and biochemical behaviours of several BREX factors (including the PglX methyltransferase, the BrxL effector, the BrxA DNA-binding protein, and a commonly-associated transcriptional regulator, BrxR), the mechanism by which BREX impedes phage replication remains largely undetermined. In this study, we identified a stable BREX sub-complex of PglZ:BrxB, generated and validated a structural model of that protein complex, and assessed the biochemical activity of PglZ from BREX, revealing it to be a metal-dependent nuclease. PglZ can cleave cyclic oligonucleotides, linear oligonucleotides, plasmid DNA and both non-modified and modified linear phage genomes. PglZ nuclease activity has no obvious role in BREX-dependent methylation, but does contribute to BREX phage defence. BrxB binding does not impact PglZ nuclease activity. These data contribute to our growing understanding of BREX phage defence.
- Department of Biosciences, Durham University, Stockton Road, DurhamDH1 3LE, UK.
Organizational Affiliation: