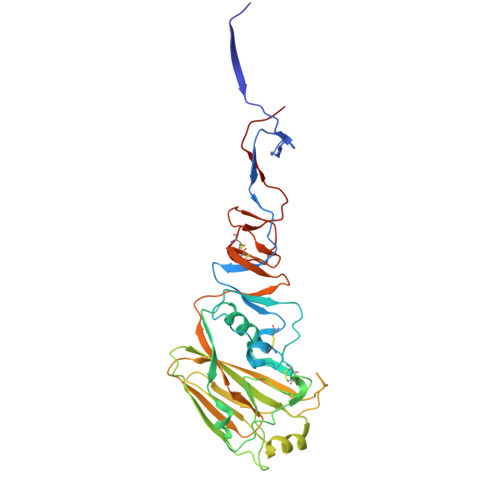
The Q226L mutation can convert a highly pathogenic H5 2.3.4.4e virus to bind human-type receptors.
Rios Carrasco, M., Lin, T.H., Zhu, X., Gabarroca Garcia, A., Uslu, E., Liang, R., Spruit, C.M., Richard, M., Boons, G.J., Wilson, I.A., de Vries, R.P.(2025) Proc Natl Acad Sci U S A 122: e2419800122-e2419800122
- PubMed: 40232794
- DOI: https://doi.org/10.1073/pnas.2419800122
- Primary Citation of Related Structures:
9NR2, 9NR5, 9NRB - PubMed Abstract:
H5Nx viruses continue to wreak havoc in avian and mammalian species worldwide. The virus distinguishes itself by the ability to replicate to high titers and transmit efficiently in a wide variety of hosts in diverse climatic environments. Fortunately, transmission to and between humans is scarce. Yet, if such an event were to occur, it could spark a pandemic as humans are immunologically naïve to H5 viruses. A significant determinant of transmission to and between humans is the ability of the influenza A virus hemagglutinin (HA) protein to shift from an avian-type to a human-type receptor specificity. Here, we demonstrate that a 2016 2.3.4.4e virus HA can convert to human-type receptor binding via a single Q226L mutation, in contrast to a cleavage-modified 2016 2.3.4.4b virus HA. Using glycan arrays, X-ray structural analyses, tissue- and direct glycan binding, we show that L133a Δ and 227Q are vital for this phenotype. Thus, whereas the 2.3.4.4e virus HA only needs a single amino acid mutation, the modified 2016 2.3.4.4b HA was not easily converted to human-type receptor specificity.
- Department of Chemical Biology & Drug Discovery, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, Utrecht 3584CG, The Netherlands.
Organizational Affiliation: