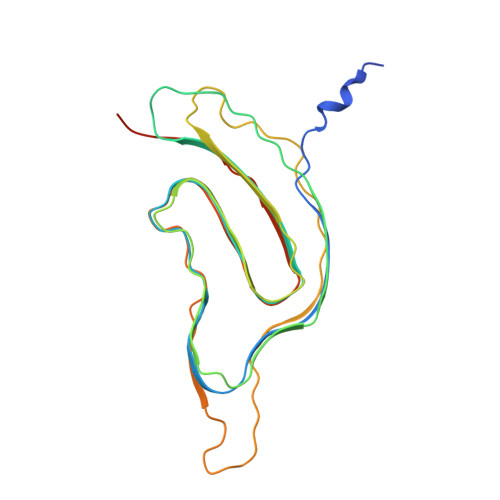
Structural basis of Pseudomonas biofilm-forming functional amyloid FapC formation.
Hansen, K.H., Golcuk, M., Byeon, C.H., Tunc, A., Plechinger, E.B., Dueholm, M.K.D., Conway, J.F., Andreasen, M., Gur, M., Akbey, U.(2025) Sci Adv 11: eadx7829-eadx7829
- PubMed: 40991694
- DOI: https://doi.org/10.1126/sciadv.adx7829
- Primary Citation of Related Structures:
9NQD - PubMed Abstract:
Biofilm-protected Pseudomonas aeruginosa causes chronic infections that are difficult to treat. FapC, the major biofilm-forming functional amyloid in Pseudomonas , is essential for biofilm integrity, yet its structural details remain unresolved. Using an integrative structural biology approach, we combine a solution nuclear magnetic resonance-based structural ensemble of unfolded monomeric FapC, a ~3.3-angstrom-resolution cryo-electron microscopy (cryo-EM) density map of FapC fibril, and all-atom molecular dynamics (MD) simulations to capture the transition from the unfolded to folded monomer to the fibrillar fold, providing a complete structural view of FapC biogenesis. Cryo-EM reveals a unique irregular triple-layer β solenoid cross-β fibril composed of a single protofilament. MD simulations initiated from monomeric and fibrillar FapC mapped structural transitions, offering mechanistic insights into amyloid assembly and disassembly. Understanding FapC reveals how Pseudomonas exploits functional amyloids for biofilm formation, and establishes a structural and mechanistic foundation for developing therapeutics targeting biofilm-related infection and antimicrobial resistance.
- Department of Structural Biology, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA.
Organizational Affiliation: