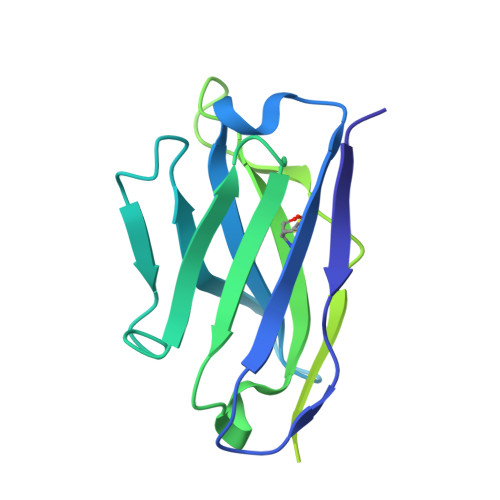
Potent neutralization of Marburg virus by a vaccine-elicited antibody.
Addetia, A., Perruzza, L., Sprouse, K., Park, Y.J., McCallum, M., Stewart, C., Partini, B., Brown, J.T., Donati, A., Culap, K., Balmelli, A., Chawla, B., Kar, S., Gazi, M., Alfson, K., Goez-Gazi, Y., Carrion Jr., R., Corti, D., Benigni, F., Veesler, D.(2026) Nature 650: 459-469
- PubMed: 41225006
- DOI: https://doi.org/10.1038/s41586-025-09868-1
- Primary Citation of Related Structures:
9NJL - PubMed Abstract:
Marburg virus (MARV) is a filovirus that causes a severe and often lethal hemorrhagic fever 1,2 . Despite the increasing frequency of MARV outbreaks, no vaccines or therapeutics are licensed for use in humans. Here, we designed mutations that improve the expression, thermostability, and immunogenicity of the prefusion MARV glycoprotein (GP) ectodomain trimer, which is the sole target of neutralizing antibodies and vaccines in development 3-8 . We discovered a fully human, pan-marburgvirus monoclonal antibody, MARV16, that broadly neutralizes all MARV isolates as well as Ravn virus and Dehong virus with 40 to 100-fold increased potency relative to previously described antibodies 9 . Moreover, MARV16 provides therapeutic protection in guinea pigs challenged with MARV. We determined a cryo-electron microscopy structure of MARV16-bound MARV GP showing that MARV16 recognizes a prefusion-specific epitope spanning GP1 and GP2, blocking receptor binding and preventing conformational changes required for viral entry. We further reveal the architecture of the MARV GP glycan cap, which shields the receptor binding site (RBS), underscoring architectural similarities with distantly related filovirus GPs. MARV16 and previously identified RBS-directed antibodies 9-11 can bind MARV GP simultaneously. These antibody cocktails require multiple mutations to escape neutralization by both antibodies, paving the way for MARV therapeutics resilient to viral evolution. MARV GP stabilization along with the discovery of MARV16 advance prevention and treatment options for MARV.
- Department of Biochemistry, University of Washington, Seattle, WA, USA.
Organizational Affiliation: