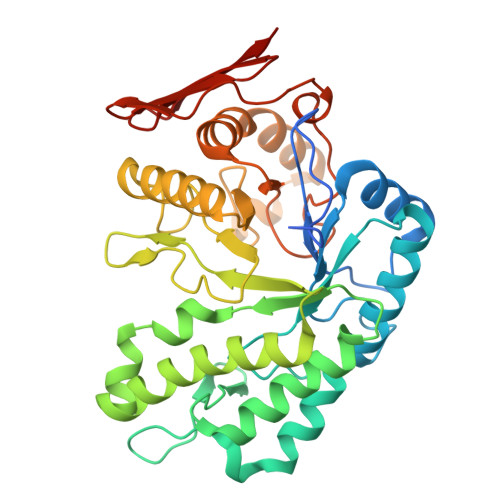
The structure of a family 168 glycoside hydrolase from the marine bacterium Muricauda eckloniae.
Knudson-Goerner, E., Boraston, A.B.(2025) Acta Crystallogr F Struct Biol Commun 81: 281-286
- PubMed: 40423696
- DOI: https://doi.org/10.1107/S2053230X2500425X
- Primary Citation of Related Structures:
9NHF - PubMed Abstract:
The genome of the marine bacterium Muricauda eckloniae sp. DK169 contains an extensive polysaccharide-utilization locus that targets fucoidan from brown algae. Within this locus is a gene that encodes a putative fucoidan-degrading glycoside hydrolase (locus tag AAY42_01205) assigned to glycoside hydrolase family 168, which we call MeGH168. We present the 2.0 Å resolution X-ray crystal structure of MeGH168, demonstrating a (β/α) 8 -barrel fold. The eight loop regions joining each α-helix and β-strand surround the catalytic groove. A comparison with the structure of a GH168, Fun168A, in complex with a fragment of fucoidan (PDB entry 8ya7) revealed conservation of key residues in the catalytic site. However, structural variation in positive-subsite loop regions may recontour the active site to create differences in substrate specificity between the two GH168s. The present data provide additional structural insights into the GH168 family, particularly expanding on sequence and structure conservation (and the lack thereof) in relation to substrate interactions.
- Biochemistry and Microbiology, University of Victoria, PO Box 1700 STN CSC, Victoria, BC V8W 2Y2, Canada.
Organizational Affiliation: