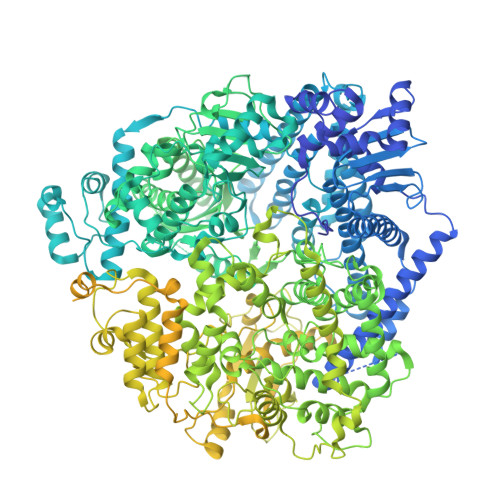
Discovery of a non-nucleoside inhibitor that binds to a novel site in the palm domain of the respiratory syncytial virus RNA-dependent RNA polymerase.
Kalin, J.H., Yin, Y., Tran, M.T., Piassek, M., Fung, A., Grosse, S., Jacoby, E., Bhaumik, A., Adhikary, S., Miller, R., Lemmens, C., Lutter, F.H., Pieters, S., Cooymans, L., Rombouts, G., Oehlrich, D., Tomaso, S., Lozada, K., Garcia, M.O., Anson, B., De Bruyn, S., Smith-Monroy, C., Neefs, J.-.M., Conceicao-Neto, N., Stoops, B., van Vlijmen, H., Patrick, A., Yu, X., Wong, V., Krosky, D., Abeywickrema, P., Mason, S., Jin, Z., Jonckers, T.H.M., Sharma, S.(2025) J Virol 99: e0017825-e0017825
- PubMed: 40454903
- DOI: https://doi.org/10.1128/jvi.00178-25
- Primary Citation of Related Structures:
9N36 - PubMed Abstract:
Respiratory syncytial virus (RSV) is a major cause of severe respiratory tract infections in infants, young children, and the elderly. We report herein the discovery and characterization of a novel RSV polymerase (RSVpol) non-nucleoside inhibitor (NNI) chemotype that binds to a previously undescribed, highly conserved site in the palm domain of the L protein. Consistent with the observed mode of inhibition, cryogenic electron microscopy (cryo-EM) revealed the site to be adjacent to the nucleotide binding site. Minireplicon assays confirmed on-target activity against RSVpol, and cell-based antiviral assays showed that the lead compound effectively inhibited viral mRNA transcription and replication in clinically relevant A and B strains. Together, our data provides valuable insights into the molecular basis of inhibition for a novel mechanism of action and paves the way for structure-based design to deliver effective therapeutics against RSV.IMPORTANCERespiratory syncytial virus (RSV) is a negative-sense, single-stranded RNA virus belonging to the family Pneumoviridae of the order Mononegavirales . Currently, monoclonal antibody treatments are only approved for infants, and vaccines are reserved for pregnant women and adults aged 60 years and older. Prophylaxis is also limited to the pediatric patient population, and there are currently no direct antiviral therapies for post-exposure treatment. Viral polymerases are considered well-validated drug targets due to their critical role in transcription and genome replication. Herein, we disclose the discovery of a spiro-indolinone series as polymerase inhibitors and describe the preliminary structure-activity relationship (SAR). A cryogenic electron microscopy (cryo-EM) structure obtained with an optimized lead revealed a novel binding site located in the palm domain, which will enable future structure-based drug design efforts. Novel RSV antivirals could be beneficial both as therapeutics following diagnosis and as a prophylactic in patients less likely to respond to vaccines.
- Janssen Research & Development, LLC, a Johnson & Johnson Company, Spring House, Pennsylvania, USA.
Organizational Affiliation: