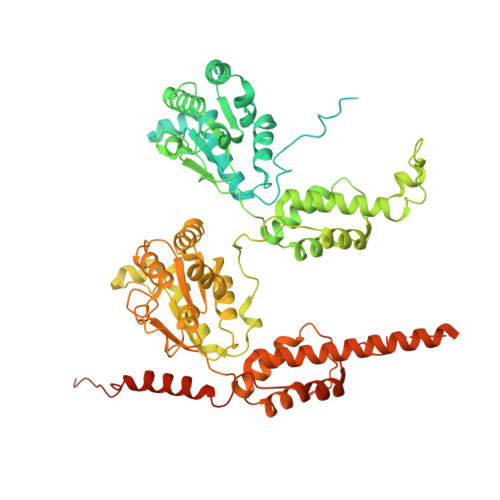
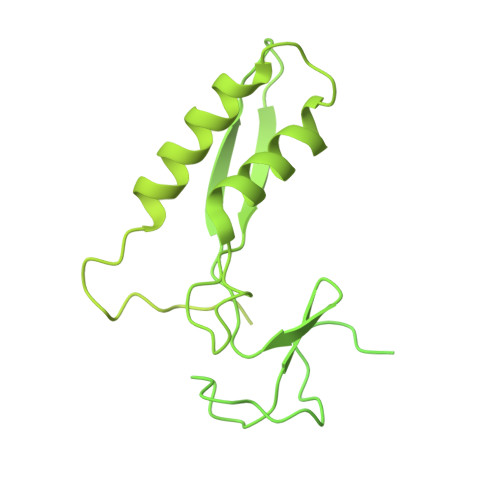
Structural insights into the coupling between VCP, an essential unfoldase, and a deubiquitinase.
Vostal, L.E., Dahan, N.E., Reynolds, M.J., Kronenberg, L.I., Kapoor, T.M.(2025) J Cell Biol 224
- PubMed: 40085114
- DOI: https://doi.org/10.1083/jcb.202410148
- Primary Citation of Related Structures:
9DIL, 9MQ6 - PubMed Abstract:
Proteostasis involves degradation and recycling of proteins from organelles, membranes, and multiprotein complexes. These processes can depend on protein extraction and unfolding by the essential mechanoenzyme valosin-containing protein (VCP) and on ubiquitin chain remodeling by ubiquitin-specific proteases known as deubiquitinases (DUBs). How the activities of VCP and DUBs are coordinated is poorly understood. Here, we focus on the DUB VCPIP1, a VCP interactor required for post-mitotic Golgi and ER organization. We determine ∼3.3 Å cryogenic electron microscopy structures of VCP-VCPIP1 complexes in the absence of added nucleotide or the presence of an ATP analog. We find that up to 3 VCPIP1 protomers interact with the VCP hexamer to position VCPIP1's catalytic domain at the exit of VCP's central pore, poised to cleave ubiquitin following substrate unfolding. We observe competition between VCPIP1 and other cofactors for VCP binding and show that VCP stimulates VCPIP1's DUB activity. Together, our data suggest how the two enzyme activities can be coordinated to regulate proteostasis.
- Laboratory of Chemistry and Cell Biology, The Rockefeller University, New York, NY, USA.
Organizational Affiliation: