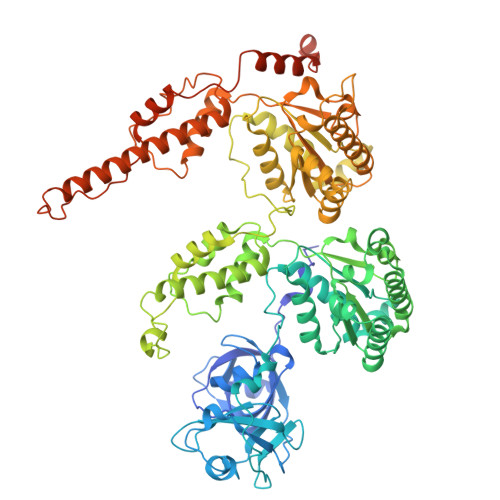
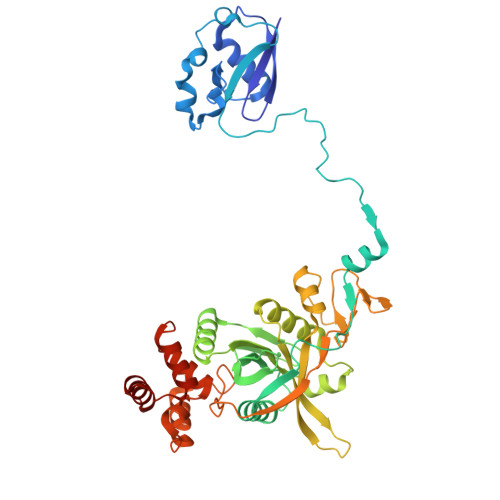
Cryo-EM structural analyses reveal plant-specific adaptations of the CDC48 unfoldase.
Huntington, B., Sandholu, A., Wang, J., Zhang, J., Zhao, L., Qureshi, B.M., Shahul Hameed, U.F., Arold, S.T.(2025) Plant Commun : 101572-101572
- PubMed: 41137397
- DOI: https://doi.org/10.1016/j.xplc.2025.101572
- Primary Citation of Related Structures:
9M3V, 9M3W, 9M3X, 9M3Y, 9M3Z, 9M4G, 9M4N - PubMed Abstract:
Targeted protein degradation through the CDC48 unfoldase enables the maintenance and rapid adaptation of proteomes across eukaryotes. However, the substantial differences among animals, fungi, and plants presumably drove extensive adaptation of CDC48-mediated degradation. Although animal and fungal CDC48 systems have shown structural and functional preservation, comparable analysis has been lacking for plants. We determined the structural and functional characteristics of Arabidopsis thaliana CDC48A in multiple states and in complex with the target-identifying cofactors UFD1 and NPL4. Our analysis revealed several features that distinguish AtCDC48A from its animal and yeast counterparts despite 80% sequence identity. Key findings include that AtCDC48A exhibits distinct domain dynamics and engages AtNPL4 in a unique manner. Moreover, AtNPL4 and AtUFD1 do not form an obligate heterodimer; instead, AtNPL4 can independently bind to AtCDC48A and mediate target degradation, although their combined action is synergistic. An evolutionary analysis indicates that these Arabidopsis features are conserved across plants and represent the ancestral state of eukaryotic CDC48 systems. Collectively, our findings suggest that plant CDC48 retains a more modular and combinatorial mode of cofactor usage, highlighting a specific adaptation of targeted protein degradation in plants.
- KAUST Center of Excellence for Smart Health, Biological and Environmental Science and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia.
Organizational Affiliation: