Structural insights into Type II-D Cas9 and its robust cleavage activity.
Wang, K., Wang, J., Yang, X., Sun, W., Sheng, G., Wang, Y.(2025) Nat Commun 16: 7396-7396
- PubMed: 40790018
- DOI: https://doi.org/10.1038/s41467-025-62128-8
- Primary Citation of Related Structures:
9LGI - PubMed Abstract:
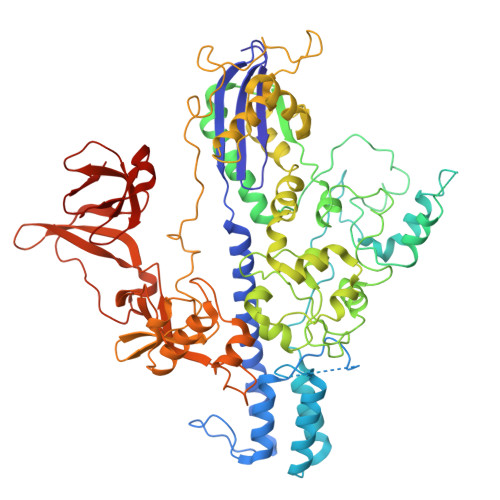
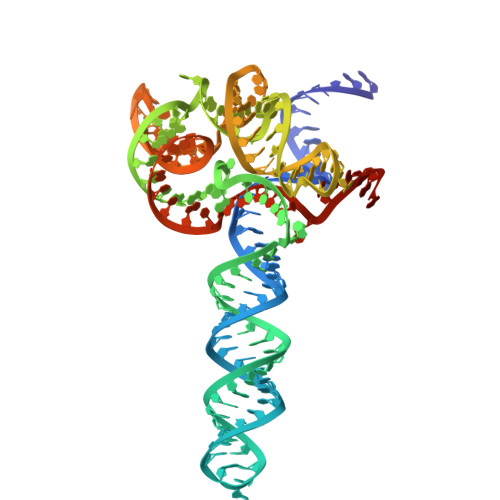
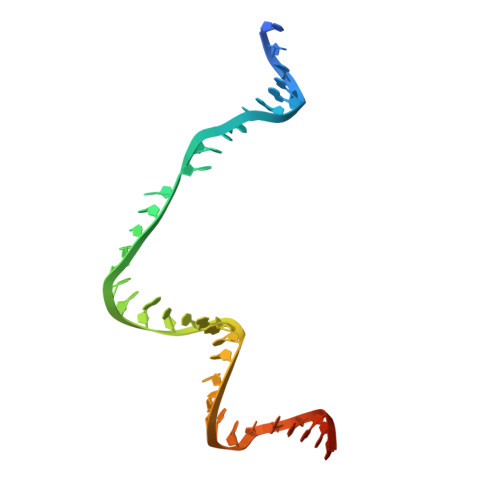
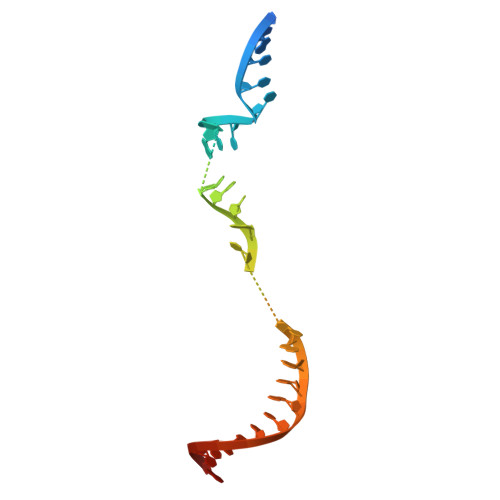
Type II-D Cas9 proteins (Cas9d) are more compact than typical Type II-A/B/C Cas9s. Here, we demonstrate that NsCas9d from Nitrospirae bacterium RBG_13_39_12 derived from a metagenomic assembly exhibits robust dsDNA cleavage activity comparable to SpCas9 in vitro. Unlike typical Cas9 enzymes that generate blunt ends, NsCas9d produces 3-nucleotide staggered overhangs. Our high-resolution cryo-EM structure of the NsCas9d-sgRNA-dsDNA complex in its catalytic state reveals the target and non-target DNA strands positioned within the HNH and RuvC catalytic pockets, respectively. NsCas9d recognizes the 5'-NRG-3' protospacer adjacent motif (PAM), with 5'-NGG-3' showing the highest cleavage efficiency. Its sgRNA structure, resembling the 5' end of IscB ωRNA, along with structural features shared with other Cas9 variants, suggests that Cas9d are hypothesized to resemble evolutionary intermediates between other Cas9 sub-types and IscB. These findings deepen our understanding of Cas9 evolution and mechanisms, highlighting NsCas9d as a promising genome-editing tool due to its compact size, DNA cleavage pattern, and efficient PAM recognition.
- State Key Laboratory of RNA Innovation, Science and Engineering, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China.
Organizational Affiliation: