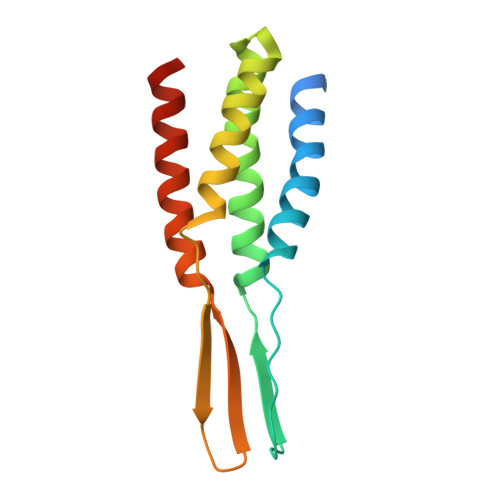
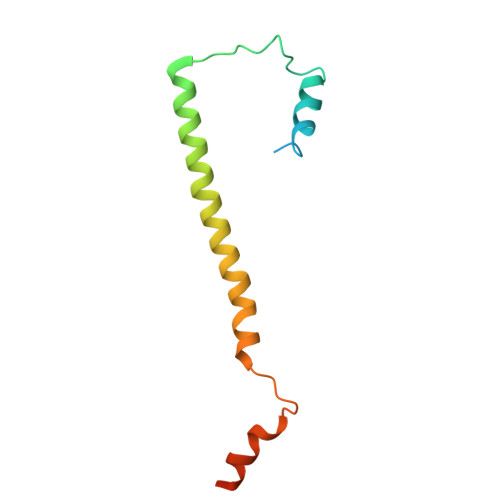
Filamentous structure of the CotVW complex, the crust proteins of the Bacillus subtilis endospore.
Jo, E., Kim, D., Baek, Y., Park, M., Lee, H., Ha, N.C.(2025) J Biological Chem 301: 110714-110714
- PubMed: 40945727
- DOI: https://doi.org/10.1016/j.jbc.2025.110714
- Primary Citation of Related Structures:
9LGH - PubMed Abstract:
The endospores of Bacillus subtilis are encased in a multilayered protective structure comprising core, cortex, inner and outer coats, and an outermost crust. Among the proteins required for crust formation, CotV and CotW are unique to B. subtilis and are hypothesized to be instrumental in maintaining spore surface integrity. However, their structural organization and functional mechanisms remain unclear. This study determined the cryogenic electron microscopy (cryo-EM) structure of the CotVW complex and revealed its filamentous helical architecture. Structural analysis showed that CotVW possesses a negatively charged surface that enables pH-dependent binding interactions. Specifically, at pH 6.0, CotVW engages in electrostatic interactions with histidine and positively charged residues, suggesting a potential regulatory mechanism influenced by the environmental pH. Our results elucidate the molecular basis of CotVW function in B. subtilis spore crust formation, highlighting its role in spore surface organization. This study advances our understanding of the spore coat architecture and may inform future research on bacterial spore resilience and structural adaptation.
- Research Institute of Agriculture and Life Sciences, Department of Agricultural Biotechnology, CALS, Seoul National University, Seoul 08826, Republic of Korea.
Organizational Affiliation: