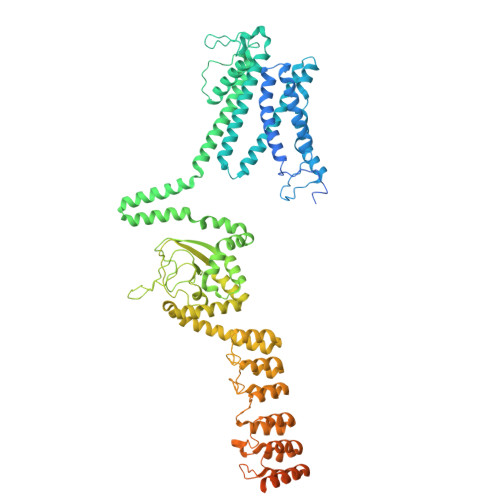
Structure reveals a regulation mechanism of plant outward-rectifying K + channel GORK by structural rearrangements in the CNBD-Ankyrin bridge.
Yamanashi, T., Muraoka, Y., Furuta, T., Kume, T., Sekido, N., Saito, S., Terashima, S., Yokoyama, T., Tanaka, Y., Miyamoto, A., Sato, K., Ito, T., Nakazawa, H., Umetsu, M., Tanudjaja, E., Tsujii, M., Dreyer, I., Schroeder, J.I., Ishimaru, Y., Uozumi, N.(2025) Proc Natl Acad Sci U S A 122: e2500070122-e2500070122
- PubMed: 40699930
- DOI: https://doi.org/10.1073/pnas.2500070122
- Primary Citation of Related Structures:
9L9U, 9LA0, 9LA1, 9LA2, 9LA3, 9LA7 - PubMed Abstract:
Guard cells, which regulate stomatal apertures in plants, possess a sophisticated mechanism for regulating turgor pressure. The outward-rectifying "K + out " channel GORK, expressed in guard cells of the plant Arabidopsis thaliana , is a central component that promotes stomatal closure by releasing K + to the extracellular space, thereby lowering turgor pressure. To date, the structural basis underlying the regulation of the K + transport activity of GORK is unclear. Using cryo-EM, we determined the structures of the GORK outward-rectifying K + channel with a resolution of 3.16 to 3.27 Å in five distinct conformations that differ significantly in their C-terminal cyclic nucleotide binding domain (CNBD) and ankyrin repeat (ANK) domain. The C-linker connects the transmembrane domains to the C-terminal domains, i.e., CNBD, CNBD-Ankyrin bridge, and ANK. The structural changes and interactions in the C-linker determine whether the closed state of GORK is closer to the preopen state or in a more removed state from the open state of the channel. In particular, interconversion in the short sequence within the CNBD-Ankyrin bridge plays a decisive role in this determination. This region forms an α-helix in the preopened state, while it adopts a nonhelical structure in further distant closed states. The dynamics of the cytosolic region strongly suggest that the K + channel activity of GORK is regulated by cytosolic signaling factors during stomatal closure.
- Department of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Sendai 980-8579, Japan.
Organizational Affiliation: