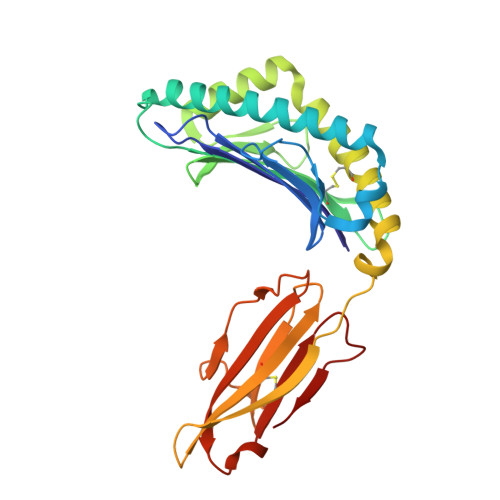
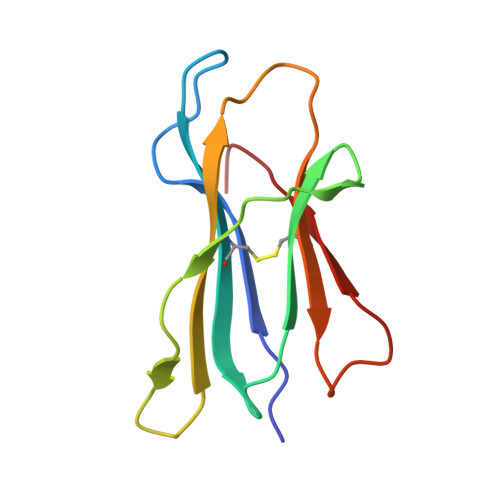
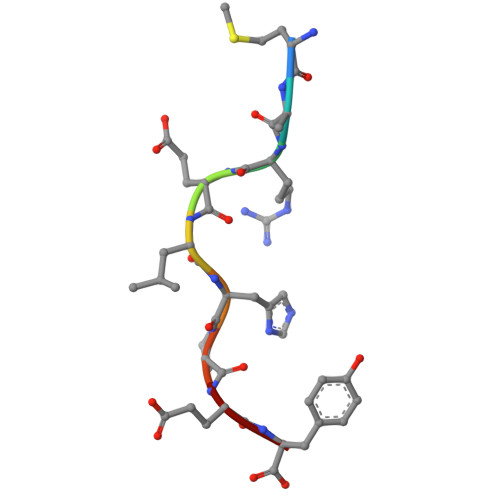
Biochemical and structural insights into position 97 micropolymorphisms in human leukocyte antigen (HLA)-C*12 allotypes and their differential disease associations.
Yang, M., Zhong, P., Liu, Q., Jiao, H., Lei, J., Wei, P.(2025) Int J Biol Macromol 306: 141681-141681
- PubMed: 40044006
- DOI: https://doi.org/10.1016/j.ijbiomac.2025.141681
- Primary Citation of Related Structures:
9L47, 9L48, 9L49, 9L4A - PubMed Abstract:
Micropolymorphisms drastically shape the antigen presentation characteristics of human leukocyte antigen class I (HLA-I) molecules, with profound implications for immune responses and disease susceptibility. HLA-C*12:02 and HLA-C*12:03 are closely related HLA-I allotypes that differ by a single amino acid substitution (R97W) but exhibit distinct associations with disease. HLA-C*12:02 has been shown to provide protective effects against HIV infection, playing a crucial role in controlling viral replication and slowing disease progression, whereas HLA-C*12:03 is associated with increased susceptibility to psoriasis. We determined the X-ray crystal structures of the two allotypes presenting MARELHPEY (MY9) and RAFPGLRYV (RV9). Peptide residues that function as anchors, as well as those accessible for T-cell antigen receptor (TCR) contact, were identified. Our results, combined with those of biochemical studies, demonstrated that the R97W variation alters the peptide-binding groove (PBG) volume and charge, leading to conformational and stability changes in pHLA-C*12 complexes and ultimately affecting peptide-binding preferences for the two HLA-C*12 allotypes. This research not only advances our understanding of the impact of HLA-I micropolymorphisms but also offers clues for the use of structure-guided therapeutics to interfere with peptide binding.
- Guangxi Key Laboratory of Special Biomedicine, School of Medicine, Guangxi University, Nanning 530004, China.
Organizational Affiliation: