Mutant of a deep sea bacterial PET hydrolase MtCut
Shanshan, L., Wei, L., Lijuan, L., Yang, J.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Engineered PET hydrolase MtCutM9 | 270 | Marinactinospora thermotolerans DSM 45154 | Mutation(s): 0 EC: 3.1.1.101 (UniProt), 3.1.1.74 (UniProt) | 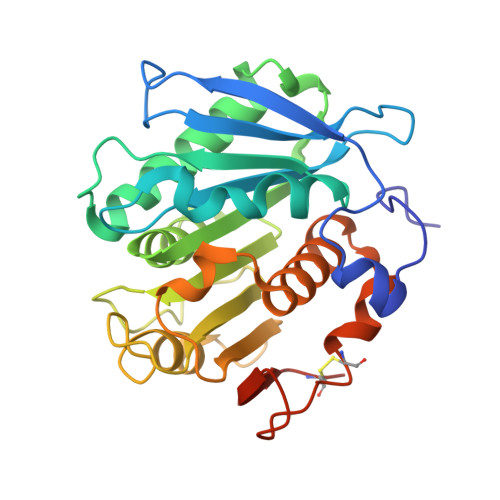 | |
UniProt | |||||
Find proteins for A0A1T4KK94 (Marinactinospora thermotolerans DSM 45154) Explore A0A1T4KK94 Go to UniProtKB: A0A1T4KK94 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A1T4KK94 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 187.94 | α = 90 |
| b = 187.94 | β = 90 |
| c = 132.02 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| Aimless | data scaling |
| MOLREP | phasing |
| xia2 | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |