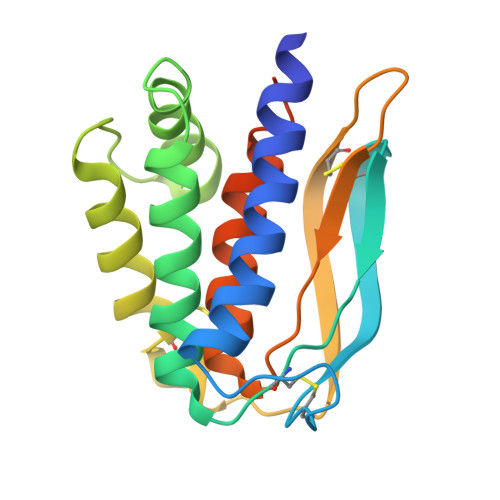
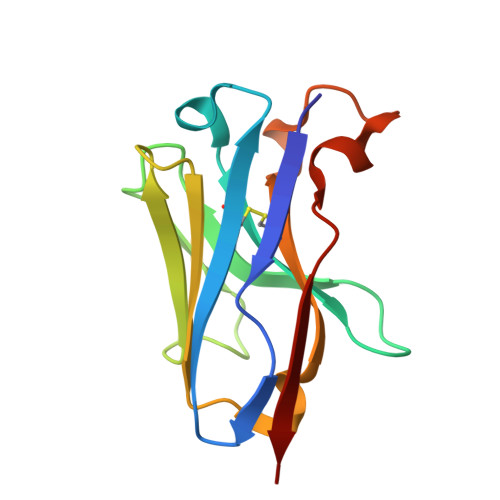
Identification of neutralizing nanobodies protecting against poxvirus infection.
Yang, X., Guo, L., Duan, H., Fan, M., Xu, F., Chi, X., Pan, S., Liu, X., Zhang, X., Gao, P., Zhang, F., Wang, X., Guo, F., Ge, J., Ren, L., Yang, W.(2025) Cell Discov 11: 31-31
- PubMed: 40133273
- DOI: https://doi.org/10.1038/s41421-025-00771-7
- Primary Citation of Related Structures:
9J7Y - PubMed Abstract:
An outbreak of mpox has triggered concerns regarding the adequacy of intervention strategies. Passive immunity conferred by neutralizing antibodies exhibits potential in the prophylaxis and treatment of orthopoxvirus infections. Despite this, the investigations of effective antibody therapeutics have been hindered by the varied nature of orthopoxvirus envelope proteins and the intricate mechanisms underpinning viral invasion. Our study involves the production of six mpox virus (MPXV) envelope proteins, which are relatively conservative and considered to play a role in the neutralization process. We employed a synthetic nanobody (Nb) library to derive a broad array of specific Nbs against these viral proteins. We identified a cross-reactive Nb, termed M1R-01, which targets the M1R protein and effectively neutralizes both vaccinia virus (VACV) and MPXV. Notably, the M1R-01-based antibody strategy provided optimal protection against a lethal VACV challenge in mice. Additionally, we determined the crystal structure of the M1R-Nb complex, uncovering novel binding attributes of M1R-01 and detailed conformational epitope information. This work provides a promising candidate for the therapy and prophylaxis of orthopoxvirus infections.
- Key Laboratory of Pathogen Infection Prevention and Control (Ministry of Education), National Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
Organizational Affiliation: