The crystal structure of RipG4 LRR domain
Jung, E.H., Kim, M.S.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| GALA protein | 360 | Ralstonia solanacearum UW551 | Mutation(s): 0 Gene Names: QQO21_16795 | 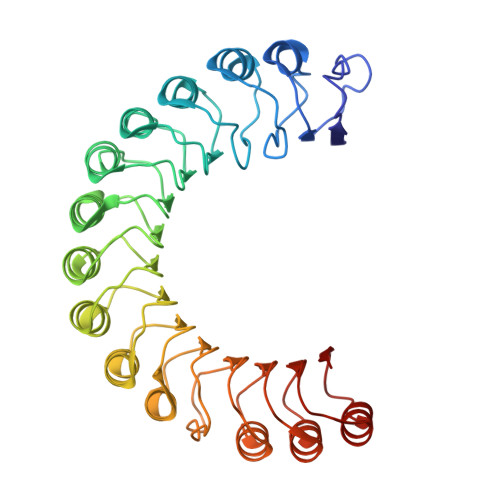 | |
UniProt | |||||
Find proteins for F5CT18 (Ralstonia solanacearum) Explore F5CT18 Go to UniProtKB: F5CT18 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | F5CT18 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 188.846 | α = 90 |
| b = 188.846 | β = 90 |
| c = 108.239 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |