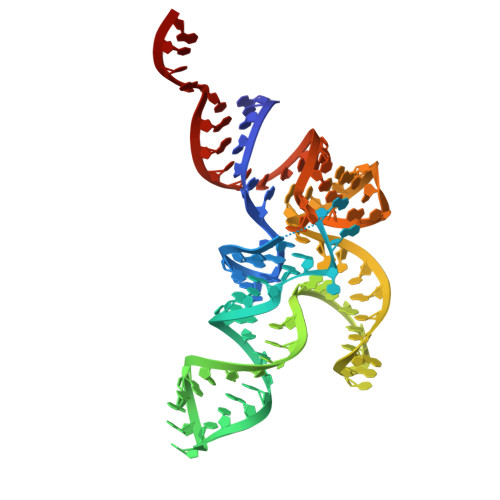
Crystal structures of free-state tRNA Leu reveal conformational flexibility of type-II tRNAs.
Cho, G., Yoo, J., Shin, K., Lee, J., Lim, J., Kim, J.(2025) RNA 31: 1305-1319
- PubMed: 40562537
- DOI: https://doi.org/10.1261/rna.080596.125
- Primary Citation of Related Structures:
9J4N, 9J4O - PubMed Abstract:
Transfer RNAs (tRNAs) are classified into Type-I and Type-II based on the length of their variable loops, with Type-II characterized by an extended variable loop. While structures of Type-I tRNAs have been well-documented, standalone structures of Type-II tRNAs have not been reported. Here, we present the first crystal structures of two free-state Type-II tRNAs, specifically tRNALeu from Bacillus subtilis and Escherichia coli. Our structures reveal that the B. subtilis tRNALeu anticodon stem-loop (ASL) retains its canonical conformation. The variable loops in both structures are well-defined, displaying a distinctive tetranucleotide loop conformation. Comparisons with Type-I tRNA and biomolecule-bound tRNAs highlight the flexibility of the ASL, variable loop, and terminal CCA residues in Type-II tRNAs, suggesting that this structural plasticity is crucial for their biological interactions and function. These findings provide new insights into the structural dynamics and functional roles of Type-II tRNAs.
- 65419 Gwangju Institute of Science and Technology Gwangju Institute of Science and Technology.
Organizational Affiliation: