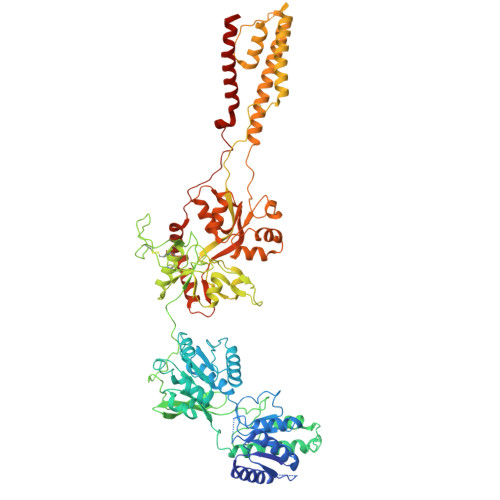
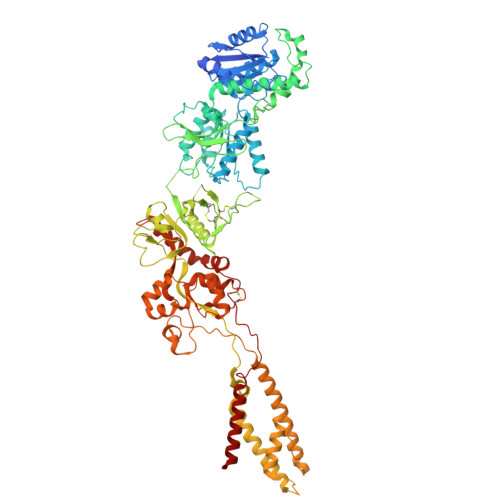
Structural insights into the diverse actions of magnesium on NMDA receptors.
Huang, X., Sun, X., Wang, Q., Zhang, J., Wen, H., Chen, W.J., Zhu, S.(2025) Neuron 113: 1006
- PubMed: 40010346
- DOI: https://doi.org/10.1016/j.neuron.2025.01.021
- Primary Citation of Related Structures:
9IYP, 9IYQ - PubMed Abstract:
Magnesium (Mg 2+ ) is a key regulatory ion of N-methyl-ᴅ-aspartate (NMDA) receptors, including conferring them to function as coincidence detectors for excitatory synaptic transmission. However, the structural basis underlying the Mg 2+ action on NMDA receptors remains unclear. Here, we report the cryo-EM structures of GluN1-N2B receptors and identify three distinct Mg 2+ -binding pockets. Specifically, site Ⅰ is located at the selectivity filter where an asparagine ring forms coordination bonds with Mg 2+ and is responsible for the voltage-dependent block. Sites Ⅱ and Ⅲ are located at the N-terminal domain (NTD) of the GluN2B subunit and involved in the allosteric potentiation and inhibition, respectively. Site Ⅱ consists of three acidic residues, and the combination of three mutations abolishes the GluN2B-specific Mg 2+ potentiation, while site Ⅲ overlaps with the Zn 2+ pocket, and mutations here significantly reduce the inhibition. Our study enhances the understanding of multifaceted roles of Mg 2+ in NMDA receptors and synaptic plasticity.
- Institute of Neuroscience, CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai 200031, China; Department of Neurology and Institute of Neurology of First Affiliated Hospital, Institute of Neuroscience, Fujian Key Laboratory of Molecular Neurology, Fujian Medical University, Fuzhou, Fujian 350005, China.
Organizational Affiliation: