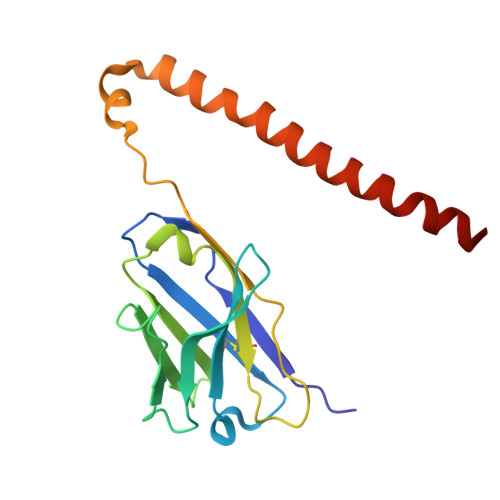
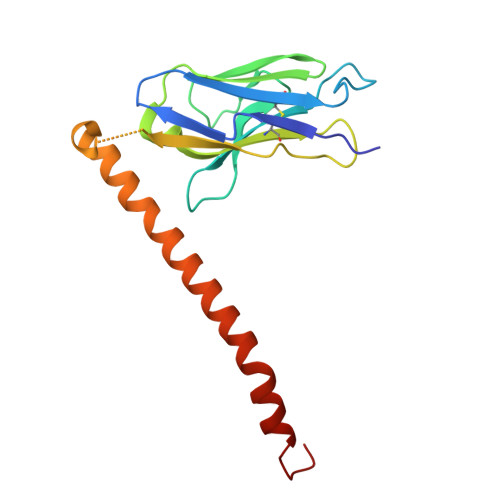
Preferential tumor targeting of HER2 by iPSC-derived CAR T cells engineered to overcome multiple barriers to solid tumor efficacy.
Hosking, M.P., Shirinbak, S., Omilusik, K., Chandra, S., Kaneko, M.K., Gentile, A., Yamamoto, S., Shrestha, B., Grant, J., Boyett, M., Cardenas, D., Keegan, H., Ibitokou, S., Pavon, C., Mizoguchi, T., Ihara, T., Nakayama, D., Abujarour, R., Lee, T.T., Clarke, R., Goodridge, J., Peralta, E., Maeda, T., Takagi, J., Arimori, T., Kato, Y., Valamehr, B.(2025) Cell Stem Cell 32: 1087-1101.e4
- PubMed: 40472844
- DOI: https://doi.org/10.1016/j.stem.2025.05.007
- Primary Citation of Related Structures:
9IUT - PubMed Abstract:
Chimeric antigen receptor (CAR) T cell therapies in solid tumors have been limited by on-target, off-tumor toxicity, antigen heterogeneity, and an inability to simultaneously overcome multiple diverse resistance mechanisms within the tumor microenvironment that attenuate anti-tumor activity. Here, we describe an induced pluripotent stem cell (iPSC)-derived CAR T cell that combines a human epidermal growth factor receptor 2 (HER2)-targeting CAR-differentially recognizing tumor from normal cells and enabling detection of both truncated and misfolded HER2-with multiplex editing designed to address and overcome obstacles to maximize efficacy in solid tumor indications. The iPSC-derived, HER2-directed CAR T cells maintained potent HER2-specific anti-tumor activity in both in vitro and in vivo settings, with limited cytolytic targeting of HER2+ normal targets. Combination with therapeutic antibodies enabled comprehensive multi-antigen targeting through both the CAR and a high-affinity, non-cleavable CD16a Fc receptor. Additionally, specific engineering of interleukin (IL)-7R-fusion, transforming growth factor β (TGF-β)-IL-18R, and CXCR2 enabled sustained persistence, resistance to TGF-β-mediated suppression, and specific migration to the tumor.
- Fate Therapeutics, Inc., San Diego, CA, USA. Electronic address: martin.hosking@fatetherapeutics.com.
Organizational Affiliation: