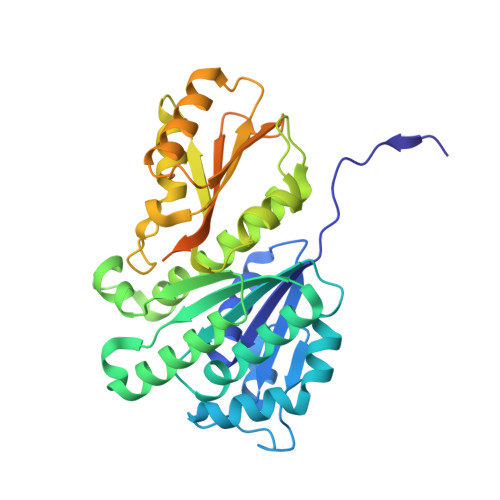
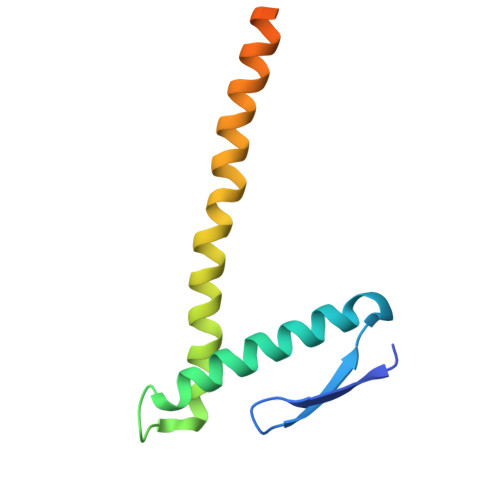
Structural basis for the interaction between the bacterial cell division proteins FtsZ and ZapA.
Fujita, J., Kasai, K., Hibino, K., Kagoshima, G., Kamimura, N., Tobita, S., Kato, Y., Uehara, R., Namba, K., Uchihashi, T., Matsumura, H.(2025) Nat Commun 16: 5985-5985
- PubMed: 40593603
- DOI: https://doi.org/10.1038/s41467-025-60940-w
- Primary Citation of Related Structures:
9ISJ, 9ISK - PubMed Abstract:
Cell division in most bacteria is regulated by the tubulin homolog FtsZ as well as ZapA, a FtsZ-associated protein. However, how FtsZ and ZapA function coordinately has remained elusive. Here we report the cryo-electron microscopy structure of the ZapA-FtsZ complex at 2.73 Å resolution. The complex forms an asymmetric ladder-like structure, in which the double antiparallel FtsZ protofilament on one side and a single protofilament on the other side are tethered by ZapA tetramers. In the complex, the extensive interactions of FtsZ with ZapA cause a structural change of the FtsZ protofilament, and the formation of the double FtsZ protofilament increases electrostatic repulsion. High-speed atomic force microscopy analysis revealed cooperative interactions of ZapA with FtsZ at a molecular level. Our findings not only provide a structural basis for the interaction between FtsZ and ZapA but also shed light on how ZapA binds to FtsZ protofilaments without disturbing FtsZ dynamics to promote cell division.
- Graduate School of Frontier Biosciences, University of Osaka, Osaka, Japan.
Organizational Affiliation: