structure of bisphosphate phosphatase at 1.64 angstroms resolution
Yu, H.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Capsule biosynthesis protein | A, B, C, D [auth E] | 134 | Phage #D | Mutation(s): 0 Gene Names: mogra_227 | 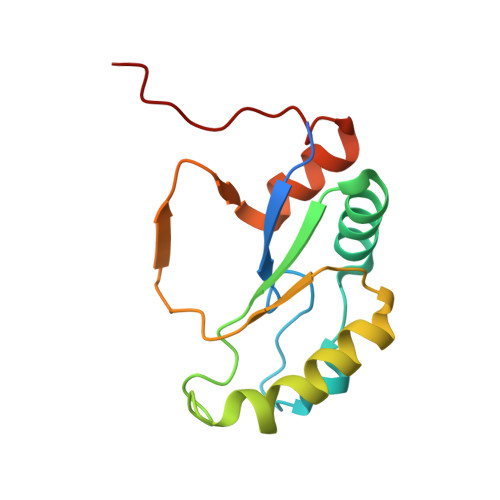 |
UniProt | |||||
Find proteins for Q7Y587 (Escherichia phage RB69) Explore Q7Y587 Go to UniProtKB: Q7Y587 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7Y587 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL (Subject of Investigation/LOI) Query on GOL | E [auth A], H [auth B], K [auth C], L [auth E] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | F [auth A], G [auth A], I [auth B], J [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 96.75 | α = 90 |
| b = 115.54 | β = 90 |
| c = 54.29 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |