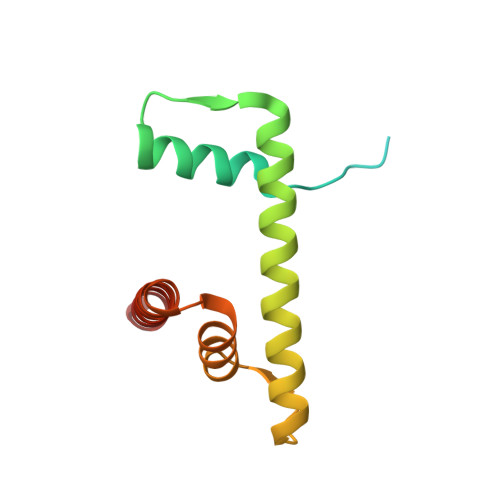
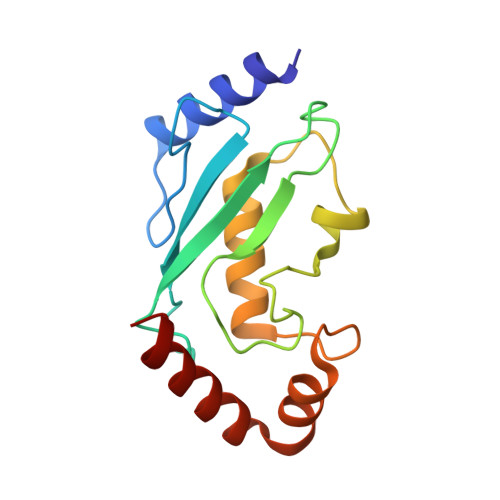
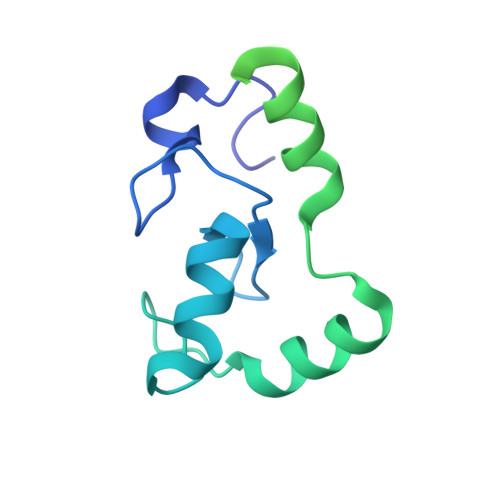
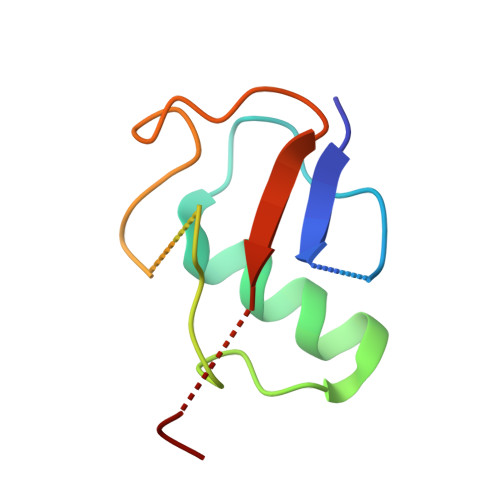
Promotion of RNF168-Mediated Nucleosomal H2A Ubiquitylation by Structurally Defined K63-Polyubiquitylated Linker Histone H1.
Shi, Q., Deng, Z., Zhang, L., Tong, Z., Li, J.B., Chu, G.C., Ai, H., Liu, L.(2025) Angew Chem Int Ed Engl 64: e202413651-e202413651
- PubMed: 39363740
- DOI: https://doi.org/10.1002/anie.202413651
- Primary Citation of Related Structures:
9IPU - PubMed Abstract:
The chemical synthesis of histones with homogeneous modifications is a powerful approach for quantitatively deciphering the functional crosstalk between different post-translational modifications (PTMs). In this study, we developed an expedient site-specific (poly)ubiquitylation strategy (CAEPL, Cysteine Aminoethylation coupled with Enzymatic Protein Ligation), which integrates the Cys-aminoethylation reaction with the process of ubiquitin-activating enzyme UBA1-assisted native chemical ligation. Using this strategy, we successfully prepared monoubiquitylated and K63-linked di- and tri-ubiquitylated linker histone H1.0 proteins, which were incorporated into individual chromatosomes. Quantitative biochemical analysis of different RNF168 constructs on H1 ubiquitylated chromatosomes with different ubiquitin chain lengths demonstrated that K63-linked polyubiquitylated H1.0 could directly stimulate RNF168 ubiquitylation activity by enhancing the affinity between RNF168 and the chromatosome. Subsequent cryo-EM structural analysis of the RNF168/UbcH5c-Ub/H1.0-K63-Ub 3 chromatosome complex revealed the potential recruitment orientation between RNF168 UDM1 domain and K63-linked ubiquitin chain on H1.0. Finally, we explored the impact of H1.0 ubiquitylation on RNF168 activity in the context of asymmetric H1.0-K63-Ub 3 di-nucleosome substrate, revealing a comparable stimulation effect of both the inter- and intra-nucleosomal crosstalk. Overall, our study highlights the significance of access to structurally defined polyubiquitylated H1.0 by the CAEPL strategy, enabling in-depth mechanistic investigations of in-trans PTM crosstalk between linker histone H1.0 and core histone H2A ubiquitylation.
- New Cornerstone Science Laboratory, Tsinghua-Peking Joint Center for Life Sciences, MOE Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology, Center for Synthetic and Systems Biology, Department of Chemistry, Tsinghua University, Beijing, 100084, China.
Organizational Affiliation: