Structural basis of human histone variant H2AZ recognized by human YL1 providing a conserved structural mechanism
Tan, W.S., Liu, Y., Hong, J.J.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H2A.Z | 98 | Homo sapiens | Mutation(s): 0 Gene Names: H2AZ1, H2AFZ, H2AZ | 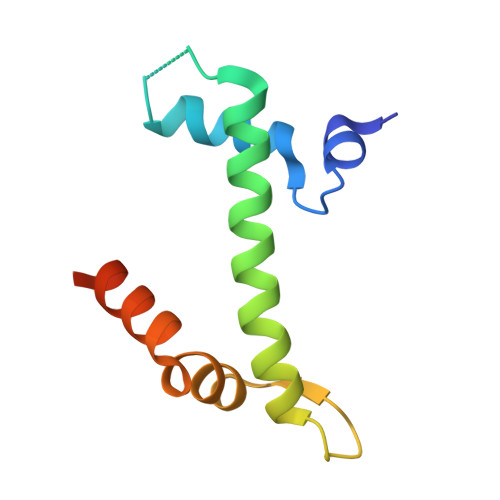 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0C0S5 (Homo sapiens) Explore P0C0S5 Go to UniProtKB: P0C0S5 | |||||
PHAROS: P0C0S5 GTEx: ENSG00000164032 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0C0S5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Histone H2B type 1-J | 94 | Homo sapiens | Mutation(s): 0 Gene Names: H2BC11, H2BFR, HIST1H2BJ | 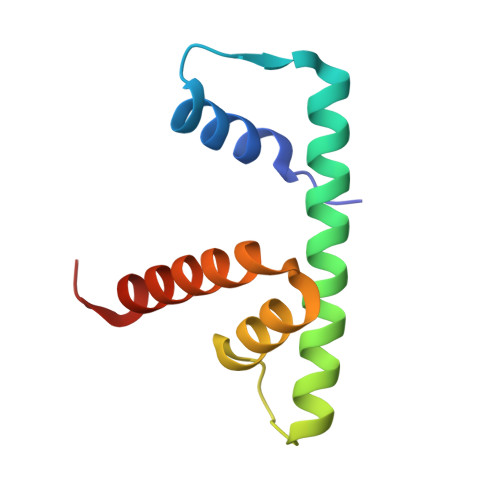 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P06899 (Homo sapiens) Explore P06899 Go to UniProtKB: P06899 | |||||
PHAROS: P06899 GTEx: ENSG00000124635 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P06899 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Vacuolar protein sorting-associated protein 72 homolog | 87 | Homo sapiens | Mutation(s): 0 Gene Names: VPS72, TCFL1, YL1 |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q15906 (Homo sapiens) Explore Q15906 Go to UniProtKB: Q15906 | |||||
PHAROS: Q15906 GTEx: ENSG00000163159 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q15906 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 116.093 | α = 90 |
| b = 126.99 | β = 90 |
| c = 70.757 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| DIALS | data reduction |
| DIALS | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 31970669 |