High conformational flexibility of phosphomannomutase 2: Implications for functioning mechanisms, stability and pharmacological chaperone design
Del Cano-Ochoa, F., Vilar, M., Vilas, A., Company, R., Perez, B., Ramon-Maiques, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Phosphomannomutase 2 | 244 | Mus musculus | Mutation(s): 0 Gene Names: Pmm2 EC: 5.4.2.8 | 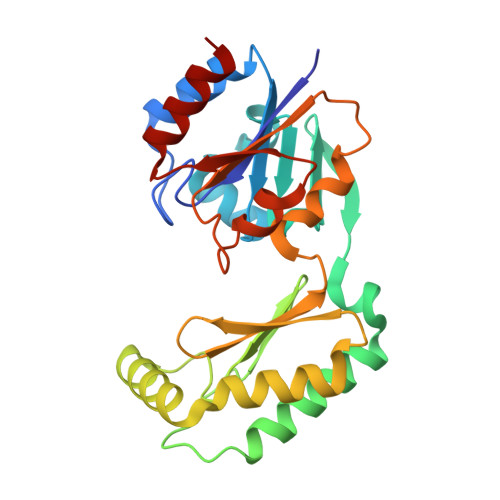 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9Z2M7 (Mus musculus) Explore Q9Z2M7 Go to UniProtKB: Q9Z2M7 | |||||
IMPC: MGI:1859214 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9Z2M7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | C [auth A], L [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | K [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| CL (Subject of Investigation/LOI) Query on CL | F [auth A], G [auth A], H [auth A], O [auth B] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| MG (Subject of Investigation/LOI) Query on MG | D [auth A], E [auth A], M [auth B], N [auth B] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| NA Query on NA | I [auth A], J [auth A], P [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 71.811 | α = 90 |
| b = 71.811 | β = 90 |
| c = 355.897 | γ = 120 |
| Software Name | Purpose |
|---|---|
| AutoProcess | data processing |
| XDS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Ministerio de Ciencia e Innovacion (MCIN) | Spain | RTI2018-098084-B-100 |
| Ministerio de Ciencia e Innovacion (MCIN) | Spain | PID2021-128468NB-I00 |